Growth promotion and biocontrol activity of Nocardiopsis dassonvillei strain YM12: an isolate from coastal agricultural land of Khambhat
Research Articles | Published: 14 October, 2019
Online ISSN : 2229-4473.
Website:www.vegetosindia.org
Pub Email: contact@vegetosindia.org
First Page: 571
Last Page: 582
Views: 2253
Keywords:
Actinobacteria:bacteria, High GC content, Liquid bioformulation, Phytohormones, Soil microbial biomass
Abstract
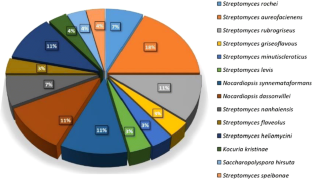
Ecological niche of coastal agricultural land of Khambhat, Gujarat (India) has an impact of marine and terrestrial process. Present study explored soil microbial biomass from a unique niche, which can contribute to the sustainable agricultural approach. Total 68 isolates with percent ratio of 42:58 of Actinobacteria:bacteria were isolated from the alkaline soil with pH 8.1. Twenty-eight isolates were identified as cultivable Actinobacteria associated with the order Streptomycetales, Streptosporengiales, micrococcales, Pseudonocardials and it belonged to 14 different genera. All the strains were screened primarily for Plant growth promoting traits like IAA production, ammonia production, phosphate as well as potassium solubilisation, glucanase and chitinase production. Strain YM12 having maximum traits positive for promoting growth and biocontrol activity was further used and characterized. Strain YM12 was identified as Nocardiopsis dassonvillei which showed 262.4 µg ml−1 IAA production, 7.2 µmol ml−1 Ammonia production along with mineral solubilisation capacity (Phosphate—288 ppm, Potassium—48.82 ppm). Apart from having plant growth promoting activity, strain showed inhibition of Fusarium growth which might be due to production of 0.004 μM ml−1 Chitinase and 0.0093 µM ml−1 Glucanase enzyme. Further it was tested for growth promotion of Pearl Millet (Pennisetum glaucum) and showed 20–30% increased growth as compared to control plants.
(*Only SPR Members can get full access. Click Here to Apply and get access)
References
- Abdulla HM (2007) Enhancement of rice straw composting by lignocellulolytic actinomycete strains. Int J Agric Biol 9:106–109
- Abdulla H (2009) Bioweathering and biotransformation of granitic rock minerals by actinomycetes abstract: characterisation of actinomycetes isolated from ancient stone and their potential for deterioration. Polish J Microbiol 57:213–220
- Agrawal T, Kotasthane AS (2012) Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. TL-1. Springerplus. 1 VN-re:73
- Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol [Internet]. 215:403–10. http://www.sciencedirect.com/science/article/pii/S0022283605803602
- Barazani OZ, Friedman J (2000) Effect of exogenously applied l-tryptophan on allelochemical activity of plant-growth-promoting rhizobacteria (PGPR). J Chem Ecol 26:343–349
- Bhatti AA, Haq S, Bhat RA (2017) Actinomycetes benefaction role in soil and plant health. Microb Pathog 111:458–467. https://doi.org/10.1016/j.micpath.2017.09.036
- Cappuccino JG, Sherman N (1992) Microbiology: a laboratory manual, 4th edn. Pearson, Harlow
- Chakraborty K (2016) Soil pH as a master variable of agricultural productivity in Burdwan-I C.D. Block, Barddhaman, West Bengal Kshudiram. Indian J Spat Sci 7:55–64
- Delvasto P, Valverde A, Ballester A, Igual JM, Muñoz JA, González F, Blázquez ML, García C (2006) Characterization of brushite as a re-crystallization product formed during bacterial solubilization of hydroxyapatite in batch cultures. Soil Biol Biochem 38:2645–2654
- Fernández LA, Zalba P, Gómez MA, Sagardoy MA (2007) Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biol Fertil Soils 43:805–809
- Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590. https://doi.org/10.1038/nrmicro.2017.87
- Germida J, Siciliano S (2001) Taxonomic diversity of bacteria associated with the roots of modern, recent and ancient wheat cultivars. Biol Fertil Soils 33:410–415. https://doi.org/10.1007/s003740100343
- Golinska P, Wypij M, Agarkar G, Rathod D, Dahm H, Rai M (2015) Endophytic actinobacteria of medicinal plants: diversity and bioactivity. Antonie van Leeuwenhoek Int J Gen Mol Microbiol 108:267–289
- Goswami D, Dhandhukia P, Patel P, Thakker JN (2014a) Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res 169:66–75
- Goswami D, Pithwa S, Dhandhukia P, Thakker JN (2014b) Delineating Kocuria turfanensis 2M4 as a credible PGPR: a novel IAA-producing bacteria isolated from saline desert. J Plant Interact 9:1–11. https://doi.org/10.1080/17429145.2013.871650
- Goswami D, Patel K, Parmar S, Vaghela H, Muley N, Dhandhukia P, Thakker JN (2015) Elucidating multifaceted urease producing marine Pseudomonas aeruginosa BG as a cogent PGPR and bio-control agent. Plant Growth Regul 75:253–263. https://doi.org/10.1007/s10725-014-9949-1
- Granér G, Persson P, Meijer J, Alström S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 224:269–276
- Intra B, Mungsuntisuk I, Nihira T, Igarashi Y, Panbangred W (2011) Identification of actinomycetes from plant rhizospheric soils with inhibitory activity against Colletotrichum spp., the causative agent of anthracnose disease. BMC Res Notes 4:1–9
- Li Q, Chen X, Jiang Y, Jiang C (2016) Morphological identification of Actinobacteria. In: Dhanasekaran DD, Jiang Y (eds) Actinobacteria—basics and biotechnological applications. InTech, Croatia
- Loucks OL (1970) Evolution of diversity, efficiency, and community stability. Am Zool 10:17–25
- Mardiah I (2019) Identification of endophytic bacterial isolated from oil palm plants with anti-fungal acitvity against Ganoderma boninense. Pharmacol Clin Pharm Res 3:41
- Meena RS, Lal R (2018) Legumes and sustainable use of soils. In: Meena RS, Das A, Yadav GS, Lal R (eds) Legum soil heal sustain manage. Springer, Singapore, pp 1–31. https://doi.org/10.1007/978-981-13-0253-4_1
- Meyer J (1976) Nocardiopsis, a new genus of the order Actinomycetazes. Int J Syst Bacteriol 26:487–493
- Palaniyandi SA, Yang SH, Zhang L, Suh JW (2013) Effects of actinobacteria on plant disease suppression and growth promotion. Appl Microbiol Biotechnol 97:9621–9636. https://doi.org/10.1007/s00253-013-5206-1
- Patel K, Goswami D, Dhandhukia P, Thakker J (2015) Techniques to study microbial phytohormones. In: Maheshwari DK (ed) Bacterial metabolites in sustainable agroecosystem. Springer International Publishing, Cham, pp 1–27. https://doi.org/10.1007/978-3-319-24654-3_1
- Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
- Rajawat MVS, Singh S, Saxena AK (2014) A new spectrophotometric method for quantification of potassium solubilized by bacterial cultures. Indian J Exp Biol 52:261–266
- Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596
- Saif S, Khan MS, Zaidi A, Ahmad E (2014) Role of phosphate-solubilizing actinomycetes in plant growth promotion: current perspective. In: Khan MS, Zaidi A, Musarrat J (eds) Phosphate solubilizing microorg princ appl microphos technol. Springer International Publishing, Cham, pp 137–156. https://doi.org/10.1007/978-3-319-08216-5_6
- Scheppler JA, Cassin PE, Gambier RM (2000) Biotechnology explorations: applying the fundamentals. ASM Press, Washington, D.C.
- Shahnewaj K, Mannan B (2013) Methods for analyzing diversity of microbial communities in natural environments. Ceylon J Sci (Bio Sci) 42:19–33
- Sheik GB, Maqbul MS, Shankar G, Ranjith MS (2017) Isolation and characterization of actinomycetes from soil of Ad-Dawadmi, Saudi Arabia and screening their antibacterial activities. Int J Pharm Pharm Sci 9:276–279
- Shutsrirung A, Chromkaew Y, Pathom-Aree W, Choonluchanon S, Boonkerd N (2013) Diversity of endophytic actinomycetes in mandarin grown in northern Thailand, their phytohormone production potential and plant growth promoting activity. Soil Sci Plant Nutr 59:322–330. https://doi.org/10.1080/00380768.2013.776935
- Singh JS, Gupta VK (2018) Soil microbial biomass: a key soil driver in management of ecosystem functioning. Sci Total Environ 634:497–500. https://doi.org/10.1016/j.scitotenv.2018.03.373
- Singh DP, Patil HJ, Prabha R, Yandigeri MS, Prasad SR (2018) Actinomycetes as potential plant growth-promoting microbial communities. Elsevier B.V., Amsterdam. http://linkinghub.elsevier.com/retrieve/pii/B9780444639875000025
- Subramanian KS, Muniraj I, Uthandi S (2016) Role of actinomycete-mediated nanosystem in agriculture. In: Subramaniam G, Arumugam S, Rajendran V (eds) Plant growth promot actinobacteria a new ave enhancing product soil fertil grain legum. Springer, Singapore, pp 233–247. https://doi.org/10.1007/978-981-10-0707-1_15
- Suryadi Y, Susilowati DN, Lestari P, Priyatno TP, Samudra IM (2014) Characterization of bacterial isolates producing chitinase and glucanase for biocontrol of plant fungal pathogens. J Agric Technol 10:983–999
- Takahashi S, Anwar MR (2007) Wheat grain yield, phosphorus uptake and soil phosphorus fraction after 23 years of annual fertilizer application to an Andosol. F Crop Res 101:160–171
- Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
- Weisany W, Raei Y, Allahverdipoor KH (2013) Role of some of mineral nutrients in biological nitrogen fixation. Bepls 2:77–84
- Yadav AN, Verma P, Kumar S, Kumar V, Kumar M, Sugitha TCK, Singh BP, Saxena AK, Dhaliwal HS (2018) Actinobacteria from rhizosphere: molecular diversity, distributions, and potential biotechnological applications. Elsevier B.V, Amsterdam. https://doi.org/10.1016/B978-0-444-63994-3.00002-3
- Zamoum M, Goudjal Y, Sabaou N, Barakate M, Mathieu F, Zitouni A (2016) Biocontrol capacities and plant growth-promoting traits of endophytic actinobacteria isolated from native plants of Algerian Sahara. J Plant Dis Prot 122:215–223
- Bakker PAHM, Pieterse CMJ, van Loon LC (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 97(2):239–243
- Bakker MG, Schlatter DC, Otto-Hanson L, Kinkel LL (2014) Diffuse symbioses: roles of plant-plant, plant-microbe and microbe-microbe interactions in structuring the soil microbiome. Mol Ecol 23(6):1571–1583
- George P, Gupta A, Gopal M, Thomas L, Thomas GV (2013) Multifarious beneficial traits and plant growth promoting potential of Serratia marcescens KiSII and Enterobacter sp. RNF 267 isolated from the rhizosphere of coconut palms (Cocos nucifera L.). World J Microbiol Biotechnol 29(1):109–117
Acknowledgements
Author Information
Department of Biotechnology, P.D. Patel Institute of Applied Sciences, Charotar University of Science and Technology, Anand, India
Department of Biotechnology, P.D. Patel Institute of Applied Sciences, Charotar University of Science and Technology, Anand, India
jankithakker.bt@charusat.ac.in