A comprehensive quality control study, marker analysis by HPTLC, HPLC and GC-MS in the course of development of quality standards for Nimbadi Churna: an ayurvedic poly herbal formulation
*Article not assigned to an issue yet
Research Articles | Published: 19 August, 2025
First Page: 0
Last Page: 0
Views: 142
Keywords: Nimbadi churna, HPTLC, HPLC, GC-MS
Abstract
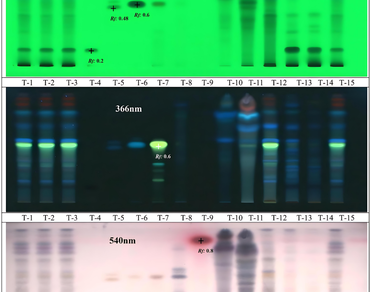
Nimbadi Churna (NC) is a classical polyherbal Ayurvedic formulation mentioned in the Ayurvedic Pharmacopoeia of India (API) and Ayurvedic Formulary of India (AFI), recommended for the treatment of kustha (skin diseases), udara (abdomen diseases), vatarakta (gout), amavata (rheumatism). The present study aimed to establish the quality standards for Nimbadi Churna with comprehensive quality control analysis by estimating markers qualitative and quantitatively. Phytochemical, aflatoxin, microbial load and elemental analysis of NC formulation were done as per standard procedures. Qualitative marker analysis was done using HPTLC and GC-MS. Quantitative marker analysis was done by HPLC. In HPTLC analysis, with a combination of toluene: ethyl acetate: formic acid (10:6:0.2 v/v/v) solvent system marker compounds piperlongumine, gallic acid, piperine, curcumin and thymol were found in NC. GC-MS analysis revealed β-asarone (32.96%), α-asarone (28.63%) in volatile oil of NC. In HPLC analysis, piperine was quantified using acetonitrile: water (80:20 v/v) gradient elution mobile phase and the amount of piperine found in NC is 0.0256%. Similarly, the amount of ellagic acid, gallic acid, piperlongumine and embelin in NC were quantified and the values are 0.0137%, 0.0117%, 0.0047% and 0.0003% respectively. The developed standard parameters for NC can be employed as a quality control tool for ensuring proper quality checks by Ayurvedic stakeholders. Nimbadi Churna, quality control, high-performance thinlayer chromatography, high-performance liquid chromatography.
References
Abhishek Verma P, Patel A, Sharma BD Kurmi (2023) Quercetin in ayurvedic formulations: High-performance thin-layer chromatography based rapid fingerprinting profiling. Phytomed Plus 3:100416. https://doi.org/10.1016/j.phyplu.2023.100416
Abraham A, Mathew L, Samuel S (2020) HPLC analysis of Pathyashadangam kwath, a classical ayurvedic polyherbal formulation. Mater Today Proc 25:115–121. https://doi.org/10.1016/j.matpr.2019.12.165
Anima Pandey N Tabbasum (2005) Study on elemental analysis, pesticides, antioxidant, marker compound and validation of conversion of Ajmodadi churna, an ayurvedic formulation into suitable dosage form. Indian J Traditional Knowl 22:537–542. https://doi.org/10.56042/ijtk.v22i3.5731
Anonymous (2008) The ayurvedic pharmacopoeia of India. Govt India Ministry Health Family Welf Department Ayurveda Yoga Naturopathy Unani Siddha Homoeopathy (AYUSH) New Delhi: Part–II Formulations, Vol–I1st ed:51–52
Bolleddu R, Narasimhaji CV, Venkatesh S, Sharma R, Mangal AK, Prasad PVV (2022) Establishment of quality parameters for fruits of Crotalaria pallida aiton. Through microscopy and phytochemical studies. Vegetos 35:622–632. https://doi.org/10.1007/s42535-022-00361-y
Brindha TR, Prabhu K, Jones S, Janaki CS, Sheriff D, Kumar HM et al (2024) The GC-MS study of the ayurvedic formulation Dhanwantharam Thailam used for rheumatism. J Pharm Bioallied Sci 16:S1829–S1832. https://doi.org/10.4103/jpbs.jpbs_14_24
Chandra H, Kumari P, Yadav S (2019) Evaluation of aflatoxin contamination in crude medicinal plants used for the Preparation of herbal medicine. Orient Pharm Exp Med 19:137–143. https://doi.org/10.1007/s13596-018-0356-4
Chavan SB, Walunj TB, Gupta VS, Deshmukh VV, Sardeshmukh SP (2024) Comparative stagewise mapping of trace elements using ICP-OES in five ayurvedic marine drugs highlights their posologic and clinical implications. J Anal Sci Technol 15:44. https://doi.org/10.1186/s40543-024-00458-w
Ibrahim S, Musthafa MM, Aslam MM, Abdurahman KM, Sudarshan M, Elumalai M et al (2021) Trace elemental fingerprinting of ayurvedic formulation Nishakatakadi using XRF and NAA. J Radioanal Nucl Chem 328:435–446. https://doi.org/10.1007/s10967-021-07659-2
Ichim MC, Haser A, Nick P (2020) Microscopic authentication of commercial herbal products in the globalized market: potential and limitations. Front Pharmacol 11:876. https://doi.org/10.3389/fphar.2020.00876
Kagathara C, Odedra K, Vadia N (2022) Development of HPTLC method for the simultaneous Estimation of quercetin, curcumin, and ascorbic acid in herbal formulations. J Iran Chem Soc 19:4129–4138. https://doi.org/10.1007/s13738-022-02586-9
Meena AK, Swathi KN, Ilavarasan R, Singh A, Bharthi V, Srikanth N (2021) Qualitative and quantitative Estimation of Diosgenin in coded ayurvedic formulation and its ingredient Trigonella foenum-graecum linn. Seeds used in diabetics. Futur J Pharm Sci 7:205. https://doi.org/10.1186/s43094-021-00354-9
Mishra A, Mishra A, K, Tiwari OP, Jha S (2016) Studies on metals and pesticide content in some ayurvedic formulations containing Bacopa monnieri L. J Integr Med 14:44–50. https://doi.org/10.1016/s2095-4964(16)60241-8
Rajkumar DB, Sriram P (2021) Evaluation of microbial load of herbal Raw materials: a necessary quality control measure to ensure safety of finished herbal preparations. Adv Biotechnol Microbiol 16:555934. https://doi.org/10.19080/AIBM.2021.16.555934
Shah NC (2005) Bharat Bhaishajya Ratnakar Volume-III. B. Jain Publishers (P) Ltd. New Delhi; pp. 172–173
Shreen DN, Eman Hammad, Mohammad AY, A (2023) Quantification of aflatoxins and health risk assessment through intake of herbal medicine formulations. Emerg Contam 9:100206. https://doi.org/10.1016/j.emcon.2023.100206
Srikanth N, Singh A, Ota S, Sreedhar B, Galib, Dhiman K S (2018) Chemical characterization of an ayurvedic herbo-mineral preparation- Mahalaxmivilas Rasa through spectroscopic & chromatographic methods. J Ayurveda Integr Med 10:262–268. https://doi.org/10.1016/j.jaim.2018.01.002
Turkson BK, Mensah MLK, Sam GH, Mensah AY, Amponsah IK, Ekuadzi E et al (2020) Evaluation of the microbial load and heavy metal content of two polyherbal antimalarial products on the Ghanaian market. Evid Based Complement Alternat Med 15:1014273. https://doi.org/10.1155/2020/1014273
Upton R, David B, Gafner S, Glasl S (2020) Botanical ingredient identification and quality assessment: strengths and limitations of analytical techniques. Phytochem Rev 19:1157–1177. https://doi.org/10.1007/s11101-019-09625-z
Vinothkanna A, Prathiviraj R, Sivakumar TR, Ma Y, Sekar S (2023) GC–MS and network Pharmacology analysis of the ayurvedic fermented medicine, Chandanasava, against chronic kidney and cardiovascular diseases. Appl Biochem Biotechnol 195:2803–2828. https://doi.org/10.1007/s12010-022-04242-7
Author Information
Central Ayurveda Research Institute, CCRAS, Ministry of Ayush, Jhansi, India