Allopathic potential of essential oil extracts on weeds germination and seedlings growth in sustainable agriculture: the phytochemical study of Tunisia’s two Melaleucaspecies
Research Articles | Published: 06 February, 2023
First Page: 165
Last Page: 172
Views: 3377
Keywords: Essential oil, n Melaleuca rugulosa Craven, n Melaleuca ericifolia Sm, 1,8-cineole, Phytotoxicity
Abstract
In continuation of the search of eco-friendly and safe alternatives for chemical pesticide for weeds management, essential oils (EOs) may be a good candidate. The current work conducted on the chemical composition of EOs extracted from leaves of two Melaleuca species: Melaleuca ericifolia Sm and Melaleuca rugulosa Craven and in second step on the assessement of their phytotoxic potential against germination and seedling growth of Sinapis arvensis, Phalaris canariensis and Triticum durum in pre-emergence stage. In post emergence stage, EOs were applied by spraying at different doses and their physiological effects on membrane integrity, tissue hydration, malondialdehyde and proline contents were assessed.
Gas chromatography–mass spectrometry showed that EOs were overwhelmingly monoterpenoid in character. The oxygenated monoterpene fraction occupied (84.48–93.84%) of the total mainly represented by 1,8-cineole as the major compound (83.55 -92.35%). Despite that, M. ericifolia EOs seem to be more richen, distinguished relatively by the variability in minor components. Germination and seedling growth were strongly inhibited under EOs treatment in a rate dose-dependent way, and all tested plants displayed different degree of sensitivity toward EOs.
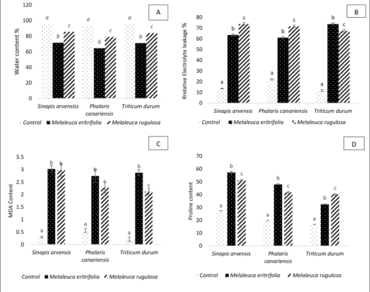
On post-emergence stage, EOs induces an irreversible phytotoxic effects, leaf chlorosis and necrosis, stem breaking and complete wilting of all tested herbs. Similarly, a decrease in relative water content, an increase of relative electrolyte leakage percentage and levels of proline and malondialdehyde contents, explain their significant phytotoxic potential.
Thus, tested Melaleuca EOs inhibited germination and growth of tested herbs in pre-emergence and post emergence stage, therefore Melaleuca EOs shows potential towards weed management. In the light of our results, M. rugulosa and M. ericifolia EOs have showed a great potential to be useful in agriculture.
References
Adams R (2005) Identification of essential Oil Components by Gas Chromatography/ Quadrupole Mass Spectroscopy. Carol Stream 16:65–120
Adetunji O, Oloke K, Bello M, Pradeep M, Jolly S (2019) Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ Technol Innov 13:304–317. https://doi.org/10.1016/j.eti.2018.12.006
Amri I, Hamrouni L, Hanana M, Jamoussi B (2011) Chemical composition of Juniperus oxycedrus L. subsp macrocarpa essential oil and study of their herbicidal effects on germination and seedling growth of weeds. Asian J Appl Sci 4:771–779
Amri I, Mancini E, De Martino L, Marandino A, Lamia H, Mohsen H, Bassem J, Scognamiglio M, Reverchon E, De Feo V (2012) Chemical Composition and Biological Activities of the Essential Oils from Three Melaleuca Species Grown in Tunisia. IJMS 13, 16580–16591. https://doi.org/10.3390/ijms131216580
Amri I, Hanana M, Gargouri S, Jamoussi B, Hamrouni L (2013) Comparative study of two coniferous species (Pinus pinaster Aiton and Cupressus sempervirens L. var. Dupreziana [A. Camus] Silba) essential oils: chemical composition and biological activity. Chil J agricultural Res 73:259–266. https://doi.org/10.4067/S0718-58392013000300008
Amri I, Mancini E, De Martino L, Hamrouni L, Hanana M, Jamoussi B, Gargouri S, Scognamiglio M, De Feo V (2014a) Chemical Composition and Biological Activities of tunisian Cupressus arizonica Greene essential oils. Chem Biodivers 11:150–160. https://doi.org/10.1002/cbdv.201300191
Amri I, Hamrouni L, Hanana M, Jamoussi B, Lebdi K (2014b) Essential oils as biological alternatives to protect date palm (Phoenix dactylifera L.) against Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae). Chil J agricultural Res 74:273–279. https://doi.org/10.4067/S0718-58392014000300004
Amri I, Hanana M, Jamoussi B, Hamrouni L (2015) Chemical composition of Thuja orientalis L. essential oils and study of their allelopathic potential on germination and seedling growth of weeds. Archives Of Phytopathology And Plant Protection 48:18–27. https://doi.org/10.1080/03235408.2014.882107
Amri I, Kouki H, Mabrouk Y, Hanana M, Jamoussi B, Hamrouni L (2021) Essential oils of tunisian Pinus radiata D. Don, chemical composition and study of their herbicidal activity. Vietnam J Chem 59:247–252. https://doi.org/10.1002/vjch.202000103
Amri I, Khammassi M, Gargouri S, Hanana M, Jamoussi B, Hamrouni L, Mabrouk Y (2022) Tunisian Pine essential oils: Chemical Composition, Herbicidal and Antifungal Properties. J Essent Oil Bearing Plants 25:430–443. https://doi.org/10.1080/0972060X.2022.2084347
Bates S, Waldren P, Teare D (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Ben Ghnaya A, Hamrouni L, Amri I, Ahoues H, Hanana M, Romane A (2016) Study of allelopathic effects of Eucalyptus erythrocorys L. crude extracts against germination and seedling growth of weeds and wheat. Nat Prod Res 30:2058–2064. https://doi.org/10.1080/14786419.2015.1108973
Bouajaj S, Romane A, Benyamna A, Amri I, Hanana M, Hamrouni L, Romdhane M (2014) Essential oil composition, phytotoxic and antifungal activities of Ruta chalepensis L. leaves from high Atlas Mountains (Morocco). Nat Prod Res 28:1910–1914. https://doi.org/10.1080/14786419.2014.945085
Cordeau S, Triolet M, Wayman S, Steinberg C, Guillemin P (2016) Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot 87:44–49. https://doi.org/10.1016/j.cropro.2016.04.016
Dmitrović S, Perišić M, Stojić A, Živković S, Boljević J, Nestorović Živković J, Aničić N, Ristić M, Mišić D (2015) Essential oils of two Nepeta species inhibit growth and induce oxidative stress in ragweed (Ambrosia artemisiifolia L.) shoots in vitro. Acta Physiol Plant 37:64. https://doi.org/10.1007/s11738-015-1810-2
Farag S, Shalaby S, El-Baroty A, Ibrahim A, Ali A, Hassan M (2004) Chemical and biological evaluation of the essential oils of different Melaleuca species. Phytother Res 18:30–35. https://doi.org/10.1002/ptr.1348
Hazrati H, Saharkhiz J, Niakousari M, Moein M (2017) Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol Environ Saf 142:423–430. https://doi.org/10.1016/j.ecoenv.2017.04.041
Heath L, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Khammassi M, Mighri H, Ben Mansour M, Amri I, Jamoussi B, Khaldi A (2022) Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) Populations growing wild in Tunisia. South Afr J Bot 148:407–414. https://doi.org/10.1016/j.sajb.2022.05.021
Khedhri S, Khammassi M, Amri I, Mabrouk Y, Dhaoudi F, Gargouri S, Hnnana M, Jamoussi B, Hamrouni L (2022) Phytochemical studies on essential oils of Pinus pinaster Aiton and evaluation of their biological activities. Arab J Med Aromatic Plants 8:75–98
Klūga A, Terentjeva M, Vukovic NL, Kačániová M (2021) Antimicrobial activity and chemical composition of essential oils against pathogenic microorganisms of Freshwater Fish. Plants 10:1265. https://doi.org/10.3390/plants10071265
Metoui N, Gargouri S, Amri I, Fezzani T, Jamoussi B, Hamrouni L (2015) Activity antifungal of the essential oils; aqueous and ethanol extracts from Citrus aurantium L. Nat Prod Res 29:2238–2241. https://doi.org/10.1080/14786419.2015.1007136
Rémita S (2001) Radiation induced lipid peroxidation: factors which determine the oxidizability of lipids. Can J Physiol Pharmacol 79(2):144–153
Saoud I, Hamrouni L, Gargouri S, Amri I, Hanana M, Fezzani T, Bouzid S, Jamoussi B (2013) Chemical composition, weed killer and antifungal activities of tunisian thyme (Thymus capitatus Hoff. Et link.) Essential oils. Acta Aliment 42:417–427. https://doi.org/10.1556/AAlim.42.2013.3.15
Singh P, Batish R, Kaur S, Arora K, Kohli K (2006) Pinene inhibits growth and induces oxidative stress in roots. Ann Botany 98:1261–1269. https://doi.org/10.1093/aob/mcl213
Taha M, Amer E, Elmarsafy E, Elkady Y (2014) Adsorption of 15 different pesticides on untreated and phosphoric acid treated biochar and charcoal from water. J Environ Chem Eng 2:2013–2025. https://doi.org/10.1016/j.jece.2014.09.001
Todero I, Confortin C, Luft, Brun T, Ugalde A, Almeida T, Arnemann A, Zabot L, Mazutti A (2018) Formulation of a bioherbicide with metabolites from Phoma sp. Sci Hort 241:285–292. https://doi.org/10.1016/j.scienta.2018.07.009
Turner C (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58:339–366. https://doi.org/10.1007/BF02180062
Wang L, Liu Q, Dong X, Liu Y, Lu J (2019) Herbicide and nitrogen rate effects on weed suppression, N uptake, use efficiency and yield in winter oilseed rape (Brassica napus L.). Global Ecol Conserv 17:e00529. https://doi.org/10.1016/j.gecco.2019.e00529
Zanella S, Gavassoni L, Bacchi A, Formagio N (2015) Activity of plant extracts on the carpogenic germination and mycelial growth of Sclerotinia sclerotiorum. https://doi.org/10.1590/1808-1657000372013
Zhang Yu, Yang X, Zhu Y, Li L, Zhang Yali, Li J, Song X, Qiang S (2019) Biological control of Solidago canadensis using a bioherbicide isolate of Sclerotium rolfsii SC64 increased the biodiversity in invaded habitats. Biol Control 139:104093. https://doi.org/10.1016/j.biocontrol.2019.104093
Author Information
Laboratory of Management and Valorization of Forest Resources, National Institute of Researches on Rural Engineering, Water and Forests, Ariana, Tunisia