Amino acid composition of pigeon pea varieties through HPTLC
*Article not assigned to an issue yet
Suthar Kirankumar P., Singh Diwakar, Patel Nitin B., Chauhan Digvijay A., Katagi Rajkumar B., Lakhani Komal G.
Research Articles | Published: 18 November, 2025
First Page: 0
Last Page: 0
Views: 113
Keywords: HPTLC, Pigeon pea, Amino acid composition
Abstract
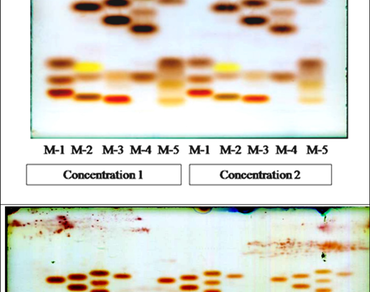
Pigeon pea (Cajanus cajan (L.) Huth) plays a pivotal role in sustaining the livelihoods of smallholder farmers in tropical and subtropical regions by contributing significantly to food and feed security, particularly as a vital source of dietary protein. Its unique amino acid profile holds immense potential for complementing cereal-based diets, thereby enabling the development of balanced protein sources and promoting nutritional security. In this study, High-Performance Thin Layer Chromatography (HPTLC) was employed to analyze the amino acid composition of twelve released pigeon pea varieties, revealing considerable genetic and nutritional variation. The optimization of chromatographic conditions showed that the mobile phase acetic acid, water, and n-butanol (4:5:1, v/v/v) gave the best separation, producing sharp, well-defined bands with very little spreading. A total of fourteen amino acids were identified, with retardation factor (Rf) values ranging from 0.05 to 0.52; among these, eight were essential and six were non-essential Among the essential amino acids, the Banas variety recorded the highest concentrations of tryptophan (11.77 mg/g) and arginine (19.69 mg/g). Additionally, AGT-2 exhibited the highest phenylalanine content (26.07 mg/g), GJP-1 had the highest lysine (6.58 mg/g), AVPP-1 the highest leucine (12.05 mg/g), GT-103 the highest histidine (9.18 mg/g) and methionine (4.10 mg/g), while GT-102 displayed the highest valine (1.36 mg/g). These substantial variations are likely attributed to the diverse genetic backgrounds and specific breeding objectives of each variety. The findings underscore the genetic potential within released pigeon pea varieties for enhancing protein quality through breeding. Varieties with superior amino acid profiles may serve as valuable parental lines in future crop improvement programs aimed at achieving a balanced amino acid composition, especially to complement cereal-based diets in protein-deficient regions.
References
Ade-Omowaye BIO, Tucker GA, Smetanska I (2015) Nutritional potential of nine underexploited legumes in south west Nigeria. Int Food Res J 22:798–806
Akande KE, Fabiyi EF (2010) Effect of processing methods on some antinutritional factors in legume seeds for poultry feeding. Int J Poult Sci 9(10):996–1001
Chen S, Wu X, Duan J, Huang P, Li T, Yin Y, Yin J (2021) Low-protein diets supplemented with glutamic acid or aspartic acid ameliorate intestinal damage in weaned piglets challenged with hydrogen peroxide. Anim Nutr 7:356–364
FAO (1973) Energy and protein requirements. Report of FAO Nutritional meeting series, No 52. Rome, Italy.
Faris DG, Singh U (1990) “Pigeon pea: nutrition and products.” In: Nene YL, Hall SD, Sheila VK (eds) The pigeon pea. CAB International, Wall-ingford, pp 401–434
Fuchs B, Süß R, Nimptsch A, Schiller J (2009) MALDI-TOF-MS directly combined with TLC: a review of the current state. Chromatographia 69:95–105
Grewal SK, Sharma KP, Bharadwaj RD, Hegde V, Sidhu SK, Singh S, Jain PK, Rasool S, Arya DK, Agrawal PK, Mondal B (2022) Characterization of chickpea cultivars and trait specific germplasm for grain protein content and amino acids composition and identification of potential donors for genetic improvement of its nutritional quality. Plant Genet Resour Characterization Util 20(6):383–393. https://doi.org/10.1017/S147926212300028X
Gupta DK, Tripathi RD, Rai UN, Dwivedi S, Mishra S, Srivastava S, Inouhe M (2006) Changes in amino acid profile and metal content in seeds of Cicer arietinum L. (chickpea) grown under various fly-ash amendments. Chemosphere 65:939–945
Kunyanga C, Imungi J, Vellingiri V (2013) Nutritional evaluation of indigenous foods with potential food-based solution to alleviate hunger and malnutrition in Kenya. J Appl Biosci 67:5277–5288
Kuraz Abebe B (2022) The dietary use of pigeon pea for human and animal diets. Sci World J 1:1–12. https://doi.org/10.1155/2022/4873008
Lakhani KG, Suthar KP, Singh D, Peddi P, Karmakar N, Patil HE (2025) Nutritional and phytochemical profiling of little millet (Panicum sumatrense Roth. ex Roem & Schult.) genotypes. J Sci Food Agric. https://doi.org/10.1002/jsfa.70216
Longvah T, Ananthan R, Bhaskara Chary K, and Venkaiah K (2017) Indian food composition tables. ICMR, New Delhi, India
Mahatma MK, Bhatnagar R, Dhandhukia P, Thakkar VR (2009) in metabolites constituent in leaves of downy mildew resistant and susceptible genotypes of pearl millet. Physiol Mol Biol Plants 15:249–255
Nestel P, Cehun M, Chronopoulos A (2004) Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am J Clin Nutr 79:390–395
Nwokolo E (1987) Nutritional evaluation of pigeon pea meal. Plant Foods Hum Nutr 37:283–290
Oomah BD (2001) Flaxseed as a functional food source. J Sci Food Agric 81(9):889–894
Otter DE (2012) Standardised methods for amino acid analysis of food. Br J Nutr 108(S2):S230–S237. https://doi.org/10.1017/S0007114512002486
Panse VG and Sukhatme PV (1967) Statistical methods for agricultural workers. Indian Council of Agricultural Research, New Delhi
Pratibha G, Srinivas I, Rao KA, Raju BMK, Thyagaraj CR, Korwar GR, Venkateswarlu B, Shanker AK, Choudhary DK, Rao KS, Srinivasarao C (2015) Impact of conservation agriculture practices on energy use efficiency and global warming potential in rainfed pigeon pea-castor systems. Eur J Agron 66:30–40. https://doi.org/10.1016/j.eja.2015.02.001
Saxena KB, Kumar RV, Rao PV (2002) Pigeon pea nutrition and its improvement. J Crop Prod 5:227–260. https://doi.org/10.1300/j144v05n01_10
Spackman DH, Stein WH, Moore S (1958) Automatic recording apparatus for use in chromatography of amino acids. Anal Chem 30(7):1190–1206
Author Information
Department of Basic Sciences, ASPEE College of Horticulture, Navsari Agricultural University, Navsari, India