Antimicrobial activity and insecticidal evaluation of Passiflora edulis Sims. (Passifloraceae)
*Article not assigned to an issue yet
Costa Adrielle Rodrigues, Menezes Saulo Almeida, Almeida-Bezerra José Weverton, de Sousa Severino Denicio Gonçalves, Antunes Dhenes Ferreira, de Oliveira Elaine Cristina Conceição, de Souza Bezerra Janaína, de Souza Dieferson Leandro, Oliveira Eveline Naiara Nuvens, de Fátima Silva de Sousa Francisca
Research Articles | Published: 01 March, 2025
First Page: 0
Last Page: 0
Views: 957
Keywords: Passion fruit. Escherichia coli. Antimicrobial. Resistance. Negative geotaxis
Abstract
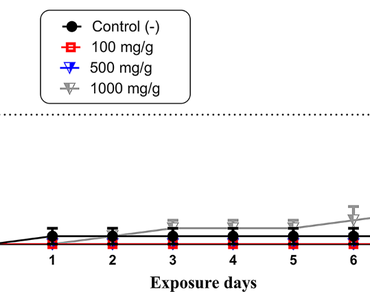
The increasing spread of bacterial resistance represents a serious threat to global health. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli are clinically relevant bacteria driving the search for new strategies to combat resistance. Plant products have emerged as promising alternatives. This study investigated the presence of phytochemical classes, toxicological activity, negative geotaxis, antibacterial activity, and the modifying effect of the ethanolic extract of Passiflora edulis (EEPe) on antibiotic action. Qualitative analyses confirmed the presence of catechins in low concentrations. In assays with Drosophila melanogaster, EEPe exhibited toxicity only at high concentrations (1000 mg/g) and showed no significant impact on negative geotaxis. Co-administration of EEPe with antibiotics significantly enhanced the efficacy of certain drugs. In P. aeruginosa, the combination with ampicillin reduced the MIC from 1024 µg/mL to 812 µg/mL. For E. coli, EEPe combined with gentamicin reduced the MIC from 256 µg/mL to 128 µg/mL, and with norfloxacin, from 512 µg/mL to 256 µg/mL. These findings suggest that EEPe may be a promising adjunct in combined antibacterial therapies, warranting further studies to elucidate its interactions and mechanisms of action.
References
Aguiar JN, de Carvalho IPSF, Domingues RAS, Maior MDCLS, Luiza VL, Barreto JOM, Tavares NUL (2023) Evolução das políticas brasileiras de saúde humana para prevenção e controle da resistência antimicrobiana: uma revisão de escopo. Pan Am J Public Health 47:e77. https://doi.org/10.26633/RPSP.2023.77
Arancibia JM (2019) Estrategias Para El uso de antibióticos en pacientes críticosstrategies for the use of antimicrobials in seriously ill patients. Revista Médica Clínica Las Condes 30(2):151–159. https://doi.org/10.1016/j.rmclc.2019.03.001
Bandara KRV, Padumadasa C, Peiris DC (2018) Potent antibacterial, antioxidant and toxic activities of extracts from Passiflora suberosa L. leaves. PeerJ 6:e4804. https://doi.org/10.7717/peerj.4804
Beressa TB, Annu A, Mtewa AG (2020) Toxicity protocols for Natural products in the drug development process. Poisonous Plants Phytochemicals Drug Discovery 189–212. https://doi.org/10.1002/9781119650034.ch9
Betts JW, Hornsey M, Higgins PG, Lucassen K, Wille J, Salguero FJ, Seifert H, La Ragione RM (2019) Restoring the activity of the antibiotic aztreonam using the polyphenol epigallocatechin gallate (EGCG) against multidrug-resistant clinical isolates of Pseudomonas aeruginosa. J Med Microbiol 68(10):1552–1559. https://doi.org/10.1099/jmm.0.001060
Borozan AB, Popescu S, Madosa E, Ciulca A, Moldovan C, Gergen I (2023) Comparative study on the antimicrobial activity of propolis, catechin, quercetin and gallic acid. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 51(2):12826–12826. https://doi.org/10.15835/nbha51212826
Boss R, Overesch G, Baumgartner A (2016) Antimicrobial Resistance of Escherichia coli, Enterococci, Pseudomonas aeruginosa, and Staphylococcus aureus from raw fish and Seafood Imported into Switzerland. J Food Prot 79(7):1240–1246. https://doi.org/10.4315/0362-028X.JFP-15-463
Clinical and Laboratory Standards Institute (CLSI) (2015) Performance Standards for Antimicrobial Susceptibility Teating; Twenty-Second Informational Supplement. I document. M100-S16CLSI. Clinical and Laboratory Standards Institute, Wayne, PA:NIH, p. 184
Clinical and Laboratory Standards Institute (CLSI) (2020) Reference method for broth dilution Antifungal susceptibility testing of yeasts; approved standard—Third Edition. CLSI document M27 A3. Wayne. PA Clinical and Laboratory Standards Institute
Costa AR, Silva JRL, de Oliveira TJS, da Silva TG, Pereira PS, Borba EFO, Brito ES, Ribeiro PRV, Almeida-Bezerra JW, Júnior JTC, Menezes IRA, Kamdem JP, Duarte AE, Barros LM (2020) Phytochemical profile of Anacardium occidentale L.(cashew tree) and the cytotoxic and toxicological evaluation of its bark and leaf extracts. South Afr J Bot 135:355–364. https://doi.org/10.1016/j.sajb.2020.09.017
Costa MS, da Silva ARP, Araújo NJS, Paulo CLR, Ribeiro PRV, Tavares JF, Pinheiro JCA, Coutinho HDM (2024) UPLC–QTOF-MS/MS analysis of saponin-enriched fractions from Calliandra Umbellifera Benth and evaluation of antibacterial activity against multidrug-resistant bacteria. Phytochem Lett 59:64–68. https://doi.org/10.1016/j.phytol.2023.11.002
Coutinho HD, Brito SM, Leite NF, Vandesmet V, Oliveira MT, Martins GM, Silva ARP, Costa MDS (2015) Avaliação comparativa da modulação de antibióticos, frente às cepas bacterianas de Escherichia coli, Staphylococcus aureus. Revista Ciencias De La Salud 13(3):345–354. https://doi.org/10.12804/revsalud13.03.2015.02
Coutinho HD, Costa JG, Lima EO, Falcão-Silva VS, Siqueira-Júnior JP (2008) Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy, 54(4), 328–330. https://doi.org/10.1159/000151267
Dadgostar P (2019) Antimicrobial resistance: implications and costs. Infection and drug resistance. 3903–3910. https://doi.org/10.2147/IDR.S234610
Dzotam JK, Touani FK, Kuete V (2016) Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma Mafaffa Lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) Gram-negative bacteria. BMC Complement Med Ther 16(9). https://doi.org/10.1186/s12906-016-0990-7
Fan FY, Sang LX, Jiang M (2017) Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 22(3):484. https://doi.org/10.3390/molecules22030484
Figueiredo D, Colomeu TC, Schumacher NSG, Stivanin-Silva LG, Cazarin CBB, Meletti LMM, Fernandes LGR, Prado MA, Zollner RL (2016) Aqueous leaf extract of Passiflora alata Curtis promotes antioxidant and anti-inflammatory effects and consequently preservation of NOD mice beta cells (non-obese diabetic). Int Immunopharmacol 35:127–136. https://doi.org/10.1016/j.intimp.2016.03.031
Foyet HS, Tsala DE, Zogo Essono Bodo JC, Carine AN, Heroyne LT, Oben EK (2014) Anti-inflammatory and anti-arthritic activity of a methanol extract from Vitellaria paradoxa stem bark. Pharmacognosy Res 7(4):367–377. https://doi.org/10.4103/0974-8490.159569
Goyal AK, Bhat M, Sharma M, Garg M, Khairwa A, Garg R (2017) Effect of green tea mouth rinse on Streptococcus mutans in plaque and saliva in children: an in vivo study. J Indian Soc Pedod Prev Dent 35(1):41–46. https://doi.org/10.4103/0970-4388.199227
Hamilton-Miller JM, Shah S (2000) Activity of the tea component epicatechin gallate and analogues against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 46(5):852–853. https://doi.org/10.1093/jac/46.5.852
Henrique HC, Macedo N, Silveira Z, Sampaio T, Silva AR, Cunha F (2018) Prospecção fitoquímica E modulação da atividade antibiótica de Cyperus rotundus L. contra bactérias multirresistentes. Revista Cubana De Plantas Medicinales, 23(2)
Houghton PJ, Howes MJ, Lee CC, Steventon G (2007) Uses and abuses of in vitro tests in ethnopharmacology: visualizing an elephant. J Ethnopharmacol 110(3):391–400. https://doi.org/10.1016/j.jep.2007.01.032
Jusuf NK, Putra IB, Dewi NK (2020) Antibacterial activity of passion fruit purple variant (Passiflora edulis Sims var. Edulis) seeds extract against Propionibacterium acnes. Clin Cosmet Invest Dermatology 13:99–104. https://doi.org/10.2147/CCID.S229743
Kumar M, Chandel M, Kaur P, Pandit K, Kaur V, Kaur S, Kaur S (2016) Chemical composition and inhibitory effects of water extract of Henna leaves on reactive oxygen species, DNA scission and proliferation of cancer cells. EXCLI J 15:842–857. https://doi.org/10.17179/excli2016-429
Mohanasundari C, Natarajan D, Srinivasan K, Umamaheswari S, Ramachandran A (2007) Antibacterial properties of Passiflora foetida L.–a common exotic medicinal plant. Afr J Biotechnol 6(23). https://doi.org/10.5897/AJB2007.000-2426
Mostefa N, Djebli N, Khanh PN, Ha NX, Anh HTN, Ha VT, Huong TT, Anh DV, Cuong NM (2023) Removed seeds of Passiflora edulis: in Vivo and in Silico studies. Chem Biodivers 20(5):e202201051. https://doi.org/10.1002/cbdv.202201051. Anti-Alzheimer’s Activity of Polyphenolic Stilbene-Rich Acetone Fraction of the Oil-
Narain N, Shanmugam S, de Souza Araújo AA (2019) Antioxidant, antimicrobial, analgesic, anti-inflammatory and antipyretic effects of bioactive compounds from Passiflora species. Medicinal plants: from farm to pharmacy, 243–274. https://doi.org/10.1007/978-3-030-31269-5_11
Nunes RGS, Pereira PS, Elekofehinti OO, Fidelis KR, da Silva CS, Ibrahim M, Barros LM, da, Cunha FAB, Lukong KE, de Menezes IRA, Tsopmo A, Duarte AE, Kamdem JP (2019) Possible involvement of transcriptional activation of nuclear factor erythroid 2-related factor 2 (Nrf2) in the protective effect of caffeic acid on paraquat-induced oxidative damage in Drosophila melanogaster. Pesticide biochemistry and physiology, 157, 161–168. https://doi.org/10.1016/j.pestbp.2019.03.017
Razzaque MS (2021) Commentary: Microbial Resistance movements: an overview of Global Public Health threats posed by Antimicrobial Resistance, and how best to counter. Front Public Health 8:629120. https://doi.org/10.3389/fpubh.2020.629120
Renzetti A, Betts JW, Fukumoto K, Rutherford RN (2020) Antibacterial green tea catechins from a molecular perspective: mechanisms of action and structure-activity relationships. Food Funct 11(11):9370–9396. https://doi.org/10.1039/d0fo02054k
Reza A, Sutton JM, Rahman KM (2019) Effectiveness of Efflux Pump Inhibitors as Biofilm disruptors and Resistance Breakers in Gram-negative (ESKAPEE) Bacteria. Antibiotics 8(4):229. https://doi.org/10.3390/antibiotics8040229
Rubio KTS, Nascimento MAPD, Martucci MEP (2022) Interações medicamentosas entre fitoterápicos padronizados pelo Sistema Único De Saúde E medicamentos convencionais. Revista Fitos 16(2):248–269. https://doi.org/10.32712/2446-4775.2022.1138
Sakalem ME, Negri G, Tabach R (2012) Chemical composition of hydroethanolic extracts from five species of the Passiflora genus. Revista Brasileira De Farmacognosia 22:1219–1232. https://doi.org/10.1590/S0102-695X2012005000108
Santos FAM, Bezerra JWA, Kamdem JP, Boligon AA, Anraku MM, da Silva ARP, Fidelis KR, Leite NF, Pinho AI, Coutinho HDM, dos Santos JEG (2019) Polyphenolic composition, antibacterial, modulator and neuroprotective activity of Tarenaya spinosa (Jacq.) Raf.(Cleomaceae). Asian Pac J Trop Biomed 9(1):12–17. https://doi.org/10.4103/2221-1691.250264
Santos TB, De Araujo FP, Neto AF, De Freitas ST, Araújo JS, Vilar SBO, Araújo AJB, Lima MS (2021) Phytochemical compounds and antioxidant activity of the pulp of two Brazilian passion fruit species: Passiflora cincinnata mast. And Passiflora edulis Sims. Int J Fruit Sci 21(1):255–269
Shrestha P, Ni J, Wong TY (2020) Synergistic and antagonistic interactions of triclosan with various antibiotics in bacteria. J Environ Sci Health Part C Toxicol Carcinog 38(3):187–203. https://doi.org/10.1080/26896583.2020.1781494
Spizzirri UG, Iemma F, Puoci F, Cirillo G, Curcio M, Parisi OI, Picci N (2009) Synthesis of antioxidant polymers by grafting of gallic acid and catechin on gelatin. Biomacromolecules 10(7):1923–1930. https://doi.org/10.1021/bm900325t
Swaminathan S, Kumar V, Kaul R (2019) Need for alternatives to animals in experimentation: an Indian perspective. Indian J Med Res 149(5):584–592. https://doi.org/10.4103/ijmr.IJMR_2047_17
Taïwe GS, Kuete V (2017) Passiflora edulis. In Medicinal spices and vegetables from Africa (pp. 513–526). Academic Press. https://doi.org/10.1016/B978-0-12-809286-6.00024-8
Valdivia AL, Fontanills YR, Álvarez LMH, Rabelo JJ, Hernández YP, Tundidor YP (2018) Propiedades fitoquímicas Y Antibacterianas De Los Extractos De las hojas de Agave fourcroydes Lem.(henequén). Revista Cubana De Plantas Medicinales, 23(2)
Viera W, Shinohara T, Samaniego I, Sanada A, Terada N, Ron L, Suárez-Tapia A, Koshio K (2022) Phytochemical composition and antioxidant activity of Passiflora spp. Germplasm Grown Ecuador Plants 11(3):328. https://doi.org/10.3390/plants11030328
Zeraik ML, Pereira CA, Zuin VG, Yariwake JH (2010) Passion fruit: a functional food. Revista Brasileira De Farmacognosia 20(3):459–471
Author Information
Department of Biological Sciences, Cariri Regional University, URCA, Crato, Brazil