Antioxidant activities of Eragrostis amabilis (L.) Wight. Arn. And Eragrostis pilosa (L.) Beauve
Research Articles | Published: 26 January, 2023
First Page: 125
Last Page: 132
Views: 3533
Keywords: Anti-oxidant, n Eragrostis amabilisn , n Eragrostis pilosan , Free radical scavenging
Abstract
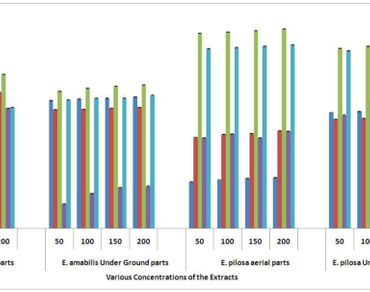
Previous studies revealed the biochemical profile, in vitro toxicity, and cytotoxic activity of Eragrostis amabilis (L.) Wight. Arn. and Eragrostis pilosa (L.) Beauve and the functional constituents of E. amabilis and E. pilosa using FT-IR. But there is no report on antioxidant activities of E. amabilis E. pilosa aerial and underground parts. With this background, the present study was undertaken to reveal the antioxidant profile of Eragrostis amabilis (L.) Wight. Arn. and Eragrostis pilosa (L.) Beauv. aerial and underground parts using the DPPH, ABTS, FRAP, SOD, metal chelating, and nitric oxide scavenging assays. A dose-dependent DPPH, ABTS, FRAP, SOD, metal chelating, and Nitric Oxide radical scavenging activity was observed in all the studied extracts of E. amabilis and E. pilosa. The aerial parts of E. pilosa and E. amabilis various extracts revealed the maximum antioxidant activities. The correlation values of total antioxidant capacity obtained by these assays were positive at p < 0.01 and (2-tailed) p < 0.05 (1-tailed) significance level, indicating that the values of antioxidant capacities assayed by NO2 assay, DPPH assay, ABTS assay, and SOD assay were highly correlative. The high rate of antioxidant and free radical scavenging potential of E. pilosa and E. amabilis are correlated with their high phenolic content occurrence and identified as a good and valuable alternative source of natural antioxidants.
References
Aiza Q, Farhan S, Muhammad TN, Abdullah IH, Bushra N, Azmat UK, Muhammad A, Huma BUA, Muhammad I (2018) Exploring the phytochemical profile of green grasses with special reference to antioxidant properties. Int J Food Prop 21(1):2566–2577. https://doi.org/10.1080/10942912.2018.1540990
Ankita C, Bhavinee D, Parni N, Neetu K, Rao B (2017) Phytochemical screening, in vitro anti quorum sensing activity and antioxidant activity of extracts of Plumeria alba, Pisonia alba and Cynodon dactylon. J Appl Pharm Sci 02162–166. https://doi.org/10.7324/JAPS.2017.70222
Ashok SA (2011) Phytochemical and pharmacological screening of Wheatgrass Juice (Triticum aestivum L.). Int J Pharm Sci Rev Res 9:159–164
Avani P, Amit P, Amit P, Patel NM (2010) Determination of polyphenols and free radical scavenging activity of Tephrosia purpurea linn leaves (Leguminosae). Pharmacognosy Res 2(3):152–158. https://doi.org/10.4103/0974-8490.65509
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99(1):191–203. https://doi.org/10.1016/j.foodchem.2005.07.042
Barroso RP, Berlim LS, Ito AS, Costa-Filho AJ (2019) In vitro antioxidant properties of golden grass (Syngonanthus nitens) by electron paramagnetic resonance. Food Sci Nutr 7(4):1353–1360. https://doi.org/10.1002/fsn3.969
Bashir A, Sultana B, Akhtar FH, Munir A, Amjad M, Hassan O (2012) Investigation on the antioxidant activity of Dheela Grass (Cyperus rotundus). Afr J Basic Appl 4(1):01–06
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 29:1199–1200. https://doi.org/10.1016/s0891-5849(98)00315-3
Carranza MSS, Linis VC, Ragasa CY, Tan MCS (2019) Chemical constituents and antioxidant potentials of seven philippine mosses malaysian. J Anal Sci 23(6):950–962. https://doi.org/10.17576/mjas-2019-2306-04
Chowdhury T, Sulthana N, Al-Mamun M, Absar N, Hasanuzzaman M (2017) From a study on the nutrients and secondary metabolites composition of two varieties of Cynodon available in Bangladesh and their anti-oxidant activities. Asian J Plant Sci Res 7(4):9–17
Ebrahimzadeh MA, Pourmorad F, Hafezi S (2008) Antioxidant activities of iranian Corn Silk. Turkish J Biol 32:43–49
El-Jemli M, Kamal R, Marmouzi I, Zerrouki A, Cherrah Y, Alaoui K (2016) Radical-Scavenging Activity and Ferric Reducing Ability of Juniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv Pharmacol Sci. https://doi.org/10.1155/2016/6392656
Ferdous UT, Yusof ZNB (2021) Medicinal prospects of Antioxidants from Algal sources in Cancer Therapy. Front Pharmacol 12:593116. https://doi.org/10.3389/fphar.2021.593116
Gebashe F, Aremu AO, Gruz J, Finnie JF, Van SJ (2020) Phytochemical profiles and antioxidant activity of grasses used in south african Traditional Medicine. Plants 9(3):371. https://doi.org/10.3390/plants9030371
Gahtori D, Chaturvedi P (2019) Bryophytes: A Potential Source of Antioxidants. In M. S. Sabovljević, & A. D. Sabovljević (Eds.), Bryophytes. IntechOpen. https://doi.org/10.5772/intechopen.84587
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13(10):572–584. https://doi.org/10.1016/s0955-2863(02)00208-5
Janakiraman N, Johnson M (2015) In Vitro antioxidant Properties of Natural Products isolated from selected species of Cyathea. J Clin Nephrol Res 2(2):1027
Janakiraman N, Johnson M, Almaida RS, Coutinho HDM (2022) In vitro antioxidant potential of Abrus percatorius L. and Asystasia gangetica (L.) T. Anderson. Lett Appl Nanobiosci 11(4):4071–4079
Janet Rani R, Sundar SK, Parthipan B, Johnson M (2013) Evaluation of Antioxidant Potential of Selected Seaweeds from South East Coast of India. Inventi Rapid Ethnopharmacology 2013; 2(1)
Johnson M, Gowtham J, Sivaraman A, Janakiraman N, Narayani M (2014) Antioxidant, Larvicidal, and cytotoxic studies on Asplenium aethiopicum (burm. f.) Becherer. Int Sch Res Notices Article ID 876170:6pages. https://doi.org/10.1155/2014/876170
Johnson M, Janakiraman N, Murugesan S (2015) Studies on antioxidant and phytochemical profiles of Leptochloa uniflora Hochst. Int Biol Biomed J Autumn 1(4):146–156
Johnson M, Janakiraman N, Murugesan S (2015) Studies on antioxidant and phytochemical profiles of Leptochloa uniflora Hochst. Int Biol Biomed J Autumn 1(4):146–156
Johnson M, Maharaja P, Janakiraman N, Adaikala Raj G, Menezes IRA, da Costa JGM, Verde LCL, Coutinho HDM (2018) Pancratium triflorum Roxb. (Amaryllidaceae) and Molineria trichocarpa (Wight) N.P. Balakr (Hypoxidaceae): cytotoxic and antioxidant activities. Food Chem Toxicol 119:290–295
Johnson M, Asha Kanimozhi S, Renisheya Joy Jeba Malar T, Shibila T, Freitas PR, Tintino SR, Menezes IRA, da Costa JGM, Coutinho HDM (2019) The antioxidative effects of bioactive products from Sargassum polycystum C.Agardh and Sargassum duplicatum J. Agardh against inflammation and other pathological issues. Complement Ther Med 46:19–23
Johnson M, Xavier Madona C, Almeida RS, Martins N, Coutinho HDM (2020) Vitro toxicity, antioxidant, anti-inflammatory and antidiabetic potential of Sphaerostephanos unitus (L.) Holttum. Antibiotics 9:333. https://doi.org/10.3390/antibiotics9060333
Johri S, Khan N (2017) In vitro antioxidant and antihaemolytic potential of Triticum aestivum Grass. Int J Complement Alt Med 9(5):00310. https://doi.org/10.15406/ijcam.2017.09.00310
Kusmardiyani S, Fitria A, Irda F (2016) Antioxidant profile and phytochemical content of three kinds of Lemon Grass grown in West Java-Indonesia. Asian J Pharm Clin Res 9(4):381–385
Manivannan V, Johnson M, Carolina A, Araújo P, Freitas HC (2022) Evaluation of antioxidant properties of Tectaria paradoxa (Fée.) Sledge and Bolbitis appendiculata (Willd.) K. Iwats Anales de Biología. 44:31–41. https://doi.org/10.6018/analesbio.44.04
Na CS, Lee MJ, Hong SS, Choi YH (2017) Antioxidant and Neuro protective activity of the aerial parts of seven Eragrostis species and bioactive compounds from E. japonica. Article Records of Natural Products 12(1):101–106. https://doi.org/10.25135/rnp.05.17.04.070
Negm W, Abo El-Seoud K, Kabbash A, El-Aasr M (2020) Investigation of the Biological Activity some Gymnosperm plants Belong to Cycadales Order. J Adv Med Pharm Res 1(1):9–13. https://doi.org/10.21608/jampr.2020.23512.1002
Ngo TN, Scarlett CJ, Bowyer MC, Ngo PD, Vuong QV (2017) Impact of different extraction solvents on Bioactive Compounds and antioxidant capacity from the Root of Salacia chinensis L. J Food Qual Article ID 9305047:8pages. https://doi.org/10.1155/2017/9305047
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Co 46:849–864. https://doi.org/10.1016/S0006-291X(72)80218-3
Patipanee K, Muangpromb A, Sukrong S (2015) Antioxidant activity and DNA protective properties of rice grass juices. Sci Asia 41:119–129. https://doi.org/10.2306/scienceasia1513-1874.2015.41.119
Pulido R, Bravo L, Sauro-Calixto F (2000) Anit-oxidant activity of dietary polyphenols as determined by a modified ferric reducing/ antioxidant power assay. J Agric Food Chem 48:3396–3402. https://doi.org/10.1021/jf9913458
Rajani M, Rasmirani R (2013) DPPH Free Radical Scavenging activity of some Leafy Vegetable sused by Tribals of Odisha, India. J Med Plants 1(1):21–27
Sansone C, Brunet C (2020) Marine Algal Antioxidants. Antioxid (Basel) 9(3):206. https://doi.org/10.3390/antiox9030206PMID: 32131430; PMCID: PMC7139763
Semerci AB, İnceçayir D, Konca T, Tunca H, Tunç K (2020) Phenolic constituents, antioxidant and antimicrobial activities of methanolic extracts of some female cones of gymnosperm plant. Indian J Biochem Biophys 57:298–303
Sivaraman A, Johnson M, Parimelazhagan T, Irudayaraj V (2013) Evaluation of antioxidant potential of ethanolic extracts of selected species of Sellaginella. Indian J Nat Prod Resour 4(3):238–244
Soler RC, Espín JC, Wichers H (2000) An easy and fast test to compare total free radical scavenger capacity of food stuffs. Phytochem Anal 11(5). https://doi.org/10.1002/1099-1565(200009/10)11
Soong YY, Barlow PJ (2004) Antioxidant activity and phenolic content of selected fruit seeds. Food Chem 88(3):411–417. https://doi.org/10.1016/j.foodchem.2004.02.003
Sujatha M, Johnson M, Vanila D, Almeida RS, Countinho HDM (2022) Biochemical profile, in vitro toxicity and cytotoxic activity of Erogrostis amabilis (L.) Wight. Arn. And Erogrostis pilosa (L.) Beauv. Lett Appl Nanobiosci 11(2):3480–3487
Sujatha M, Johnson M, Vanila D (2022) FT-IR Profile of Eragrostis amabilis (L.) Wight. Arn and Eragrostis pilosa (L.) Beauv. Romanian J. Biophys., Vol. 32, No. 3, P. 000–000, Bucharest, (Accepted)
Wolski GJ, Sadowska B, Fol M, Podsędek A, Kajszczak D, Kobylińska A (2021) Cytotoxicity, antimicrobial and antioxidant activities of mosses obtained from open habitats. PLoS ONE 16(9):e0257479. https://doi.org/10.1371/journal.pone.0257479 PMID: 34543304; PMCID: PMC8452054
Xu BJ, Chang SK (2008) Total phenolic content and antioxidant properties of eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. J Food Sci 73(2):19–27. https://doi.org/10.1111/j.1750-3841.2007.00625.x
Zielinski AA, Haminiuk CW, Alberti A, Nogueira A, Demiate IM, Granato D (2014) A comparative study of the phenolic compounds and the in vitro antioxidant activity of different brazilian teas using multivariate statistical techniques. Food Res Int 60:246–254. https://doi.org/10.1016/j.foodres.2013.09.010
Author Information
Centre for Plant Biotechnology, Department of Botany, St. Xavier’s College (Autonomous), Palayamkottai, India