Arbuscular mycorrhizal and dark septate endophytic fungal association in three wild edible fruit plants of Manipur, Northeastern India
*Article not assigned to an issue yet
Chanu Salam Nirmalashachi, Pandey Radha Raman, Kannaiah Surendirakumar
Research Articles | Published: 23 September, 2025
First Page: 0
Last Page: 0
Views: 108
Keywords: AM fungi, DSE fungi, Root colonization, n Antidesman buniusn , n Elaeagnusn confertan , n Phyllanthusn acidusn
Abstract
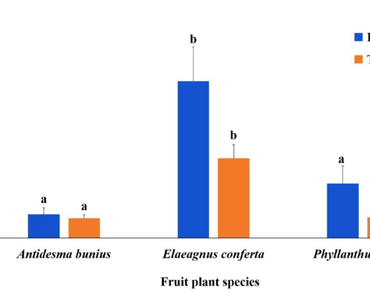
Despite the ecological importance of arbuscular mycorrhizal (AM) fungi in the growth and yield of fruit crops, little is known about the indigenous endorhizal symbionts in neglected wild edible fruit plants of subtropical biomes. Hence, we evaluated the diversity and abundance of the AM fungal communities in the rhizosphere soils, as well as colonization patterns of AM and dark septate endophytic (DSE) fungi in the roots of three fruit plant species viz. Antidesma bunius, Elaeagnus conferta and Phyllanthus acidus, collected from Manipur, Northeastern (NE) region of India. In this study, 29 AM species were isolated from both natural field as well as trap culture soils of selected fruit plants. All the examined fruit plants had dual root colonization of AM and DSE fungi and revealed Arum-Paris type AM morphology. The highest AMF root colonization (%RLTC) was recorded in P. acidus, while DSE fungi (%RLTDC) were most prevalent in A. bunius. A significant positive and negative correlation also existed among SD, some AM and DSE fungal variables and soil properties (P < 0.05). Furthermore, the current study is the first to report on the prevalence of AM and DSE fungal colonization in selected wild edible fruit plants growing in mountainous terrains, indicating the possibility of using them in the future for sustainable fruit production.
References
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications Oxford, Leipzig Germany, p 557
Bever JD, Schultz PA, Pringle A, Morton JB (2001) Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why: the high diversity of ecologically distinct species of arbuscular mycorrhizal fungi within a single community has broad implications for plant ecology. Bioscience 51(11):923–931. https://doi.org/10.1641/0006-3568(2001)051[0923:AMFMDT]2.0.CO;2
Cao MA, Wang P, Hashem A, Wirth S, Abd Allah EF, Wu QS (2021) Field inoculation of arbuscular mycorrhizal fungi improves fruit quality and root physiological activity of citrus. Agriculture (Basel) 11(12):1297. https://doi.org/10.3390/agriculture11121297
Chakraborty R, De B, Devanna SS (2012) Anti-inflammatory, antinociceptive and antioxidant activities of Phyllanthus acidus L. extracts. Asian Pac J Trop Biomed. https://doi.org/10.1016/S2221-1691(12)60343-8
Chanu SN, Pandey RR, Kannaiah S (2025) Arbuscular mycorrhizal and dark septate endophytic fungal symbioses in three different Citrus species from hillside orchards of North-eastern India. Agri Res 10:1–14. https://doi.org/10.1007/s40003-025-00870-7
Dandge PB, Kasabe PJ, Patil RM (2011) Evaluation of medicinal and nutritional components from the Elaeagnus conferta fruit. Sci Res Rep 1(2):56–60
Deb D, Sarkar A, Barma BD, Datta BK, Majumdar K (2013) Wild edible plants and their utilization in traditional recipes of Tripura, Northeast India. Adv Biol Res 7:203–211
Dickson S (2004) The Arum-Paris continuum of mycorrhizal symbioses. New Phytol 163:187–200. https://doi.org/10.1111/j.1469-8137.2004.01095.x
Fors RO, Saggin Júnior OJ, Carneiro MA, Berbara RL (2020) Selection of arbuscular mycorrhizal fungi for sugarcane in four soils with the presence of dark septate endophytes. Acta Sci Agron 42:e42477. https://doi.org/10.4025/actasciagron.v42i1.42477
Gill NS, Gupta M (2018) Elaeagnus conferta: a comprehensive review. Res J Pharm Tech 11(6):2667–2671. https://doi.org/10.5958/0974-360X.2018.00494.8
Gong MG, Ming T, Zhang QM, Feng XX (2012) Effects of climatic and edaphic factors on arbuscular mycorrhizal fungi in the rhizosphere of Hippophae rhamnoides in the Loess Plateau, China. Acta Ecol Sin 32:62–67. https://doi.org/10.1016/j.chnaes.2011.12.005
Guadarrama P, Alvarez-Sanchez FJ (1999) Abundance of arbuscular mycorrhizal fungi spores in different environments in a tropical rain forest Veracruz Mexico. Mycorrhiza 8:267–270
Hazarika TK, Singh TS (2018) Wild edible fruits of Manipur, India: associated traditional knowledge and implications to sustainable livelihood. Genet Resour Crop Evol 65:319–332. https://doi.org/10.1007/s10722-017-0534-0
He JD, Shu B, Wu QS (2020) Mycorrhizosphere of fruit crops: Nature and properties. In Fruit Crops, pp 325–338
Islary A, Sarmah J, Basumatary S (2017) Nutritional value, phtochemicals and antioxidant properties of two wild edible fruits (Eugenia operculata Roxb. and Antidesma bunius L.) from Assam, North-East India. Med J Nutrition Metab 10:29–40. https://doi.org/10.3233/MNM-16119
Jackson ML (1971) Soil Chemical Analysis. Prentice Hall, New Delhi, India, p 498
Jaison S, Rajeswari K, Muthukumar T (2012) Patterns of endorhizal fungal associations in fruit crops of southern India. J Plant Nutr Soil Sci 175:572–581. https://doi.org/10.1002/jpln.201100173
Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Tang D, Wang E (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356:1172–1175. https://doi.org/10.1126/science.aam9970
Jorjong S, Butkhup L, Samappito S (2015) Phytochemicals and antioxidant capacities of Mao-luang (Antidesma bunius L.) cultivars from Northeastern Thailand. Food Chem 181:248–255. https://doi.org/10.1016/j.foodchem.2015.02.093
Kassem MES, Hashim AN, Hassanein HM (2013) Bioactivity of Antidesma bunius leaves (Euphorbiaceae) and their major phenolic constituents. Eur Sci J 9(18):217–228
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92(4):486–488. https://doi.org/10.1016/S0953-7562(89)80195-9
Kronyut O, Sutthanut K (2019) Phenolic profile, antioxidant activity, and anti-obesogenic bioactivity of Mao luang fruits (Antidesma bunius L.). Molecules 24:4109. https://doi.org/10.3390/molecules24224109
Larbi-Koranteng S, Kankam F, Adomako J, Abdulai M (2022) Role of mycorrhizae in crop protection. In: Arbuscular Mycorrhizal Fungi in Agriculture-New Insights. Intech Open https://doi.org/10.5772/intechopen.109020
Lekberg Y, Koide RT, Rohr JR, Ablrich-Wolfe L, Morton JB (2007) Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95:95–105. https://doi.org/10.1111/j.1365-2745.2006.01193.x
Lingfei L, Anna Y, Zhiwei Z (2005) Seasonality of arbuscular mycorrhizal symbiosis and dark septate endophytes in a grassland site in southwest China. FEMS Microbiol Ecol 54(3):367–373. https://doi.org/10.1016/j.femsec.2005.04.011
Liu H, Wang Y, Tang M (2017) Arbuscular mycorrhizal fungi diversity associated with two halophytes Lycium barbarum L. and Elaeagnus angustifolia L. in Ningxia, China. Arch Agron Soil Sci 63(6):796–806. https://doi.org/10.1080/03650340.2016.1235783
Mandyam K, Jumpponen A (2005) Seeking the elusive function of root-colonizing dark septate endophytic fungi. Stud Mycol 53:173–189. https://doi.org/10.3114/sim.53.1.173
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Muthukumar T, Muthuraja R (2016) Arbuscular mycorrhizal and dark septate endophyte fungal associations in Asparagus. Turk J Bot 40:1–14. https://doi.org/10.3906/bot-1602-11
Naik SR, Nandini ML, Jameel MdA, Venkataramana KT, Mukundalakshmi L (2018) Role of arbuscular mycorrhiza in fruit crops production. Int J Pure App Biosci 6(5):1126–1133. https://doi.org/10.18782/2320-7051.7088
Ortas I (2018) Role of mycorrhizae on mineral nutrition of fruit trees. Acta Hortic. https://doi.org/10.17660/ActaHortic.2018.1217.34
Pandey RR, Chongtham I, Muthukumar T (2016) Influence of season and edaphic factors on endorhizal fungal associations in subtropical plantation forest trees of Northeastern India. Flora 222:1–12. https://doi.org/10.1016/j.flora.2016.03.011
Pandey RR, Surbala L, Srivastava AK (2020) Arbuscular mycorrhizal and dark septate endophyte fungal associations in two dominant ginger species of Northeast India. Proc Nat Acad Sci India Sect B Biol Sci 90(4):885–894. https://doi.org/10.1007/s40011-019-01159-w
Rodrigues KM and Rodrigues BF (2016) Diversity of arbuscular mycorrhizal fungi in fruit cropping system. Daya Publishing House, New Delhi, pp 3–24
Schenck NC, Perez Y (1990) Manual for the identification of VA mycorrhizal fungi. Synergistic Publications, Gainesville
Sehgal JL, Sen TK, Chamuah GS, Singh RS, Nayak DC, Saxena RK, Baruah U, Maji UK (1993) Soils of Manipur for land use planning. National bureau of soil survey and land use planning, Nagpur
Sharma BP, Handique PJ, Devi HS (2015) Antioxidant properties, physic-chemical characteristics and proximate composition of five wild fruits of Manipur. India J Food Sci Technol 52(2):894–902. https://doi.org/10.1007/s13197-013-1128-2
Siddique Z, Khan MI, Badruddeen Akhtar J, Ahmad M (2022) Therapeutic potential of star gooseberry (Phyllanthus acidus, (Skeels)) for treatment of diabetes and related complications: a review. J Diabetes Metab 13(6):939. https://doi.org/10.35248/2155-6156.1000939
Sinha S, Chakraborty K, Saha AK, Das P (2016) Mycorrhizal colonization in plants from selected home garden of Tripura. J Mycopathol Res 54(3):349–353
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. Academic Press, New York, p 787
Surendirakumar K, Chongtham I, Pandey RR (2016) Arbuscular mycorrhizal and dark septate endophyte fungal associations in three indigenous rice (Oriza sativa L.) cultivars of Manipur, North East India. J Mycopathol Res 54(3):415–422
Surendirakumar K, Chongtham I, Pandey RR, Muthukumar T (2021) Arbuscular mycorrhizal and dark septate endophytic fungal symbioses in Parkia timoriana (DC.) Merr. and Solanum betaceum Cav. plants growing in North East India. Vegetos 34:761–774. https://doi.org/10.1007/s42535-021-00258-2
Surendirakumar K, Pandey RR, Muthukumar T, Chandrasekaran M (2022) Biodiversity and application of native arbuscular mycorrhizal fungal species with rhizobacteria on growth and yield enhancements in cowpea and aromatic black rice from North Eastern India. In Microbes and Microbial Biotechnology for Green Remediation, pp 321–357
Surendirakumar K, Pandey RR, Palaniappan P, Sathish M (2023) Role of Arbuscular Mycorrhizal Symbiosis in Different Land-Use Systems of North East India: A Review. Endophytic and Arbuscular Mycorrhizal Fungi and Their Role in Sustainable Agriculture. Publisher Nova Science Publishers, Inc., USA
Tang C, Zhang Z, Yu L, Li Y (2023) Research progress of arbuscular mycorrhizal fungi promoting citrus growth. Horticulturae 9(11):1162. https://doi.org/10.3390/horticulturae9111162
van der Heijden MGA, Bardgett RD, Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Venkatachalapathi A, Nagarajan N (2021) Symbiotic relationship of AM fungi with root of Phyllanthus amarus in Western Ghats, of Tamil Nadu. Kong Res J 8(1):65–68. https://doi.org/10.26524/krj.2021.9
Wang J, Wang GG, Zhang B, Yuan Z, Fu Z, Yuan Y, Zhu L, Ma S, Zhang J (2019) Arbuscular mycorrhizal fungi associated with tree species in a planted forest of Eastern China. Forests 10:424. https://doi.org/10.3390/f10050424
Wang S, Bi Y, Quan W, Christie P (2022) Growth and metabolism of dark septate endophytes and their stimulatory effects on plant growth. Fungal Biol 126(10):674–686. https://doi.org/10.1016/j.funbio.2022.08.006
Wężowicz K, Rozpądek P, Turnau K (2017) Interactions of arbuscular mycorrhizal and endophytic fungi improve seedling survival and growth in post-mining waste. Mycorrhiza 27:499–511. https://doi.org/10.1007/s00572-017-0768-x
Author Information
Department of Life Sciences (Botany), Manipur University, Canchipur, Imphal, India