Bioactivities and metabolites profiling of different solvent extracts of novel Poly-Herbal Formulation used by tea tribes of Upper Assam
*Article not assigned to an issue yet
Dutta Aashis, Sarma Anindita, Das Manas, Tayung Kumananda, Brahma Daisy, Das Leena
Research Articles | Published: 11 December, 2025
First Page: 0
Last Page: 0
Views: 100
Keywords: Anti-oxidant, Anti-microbial, Flavonoid, Phenolic, Poly-Herbal Formulation (PHF)
Abstract
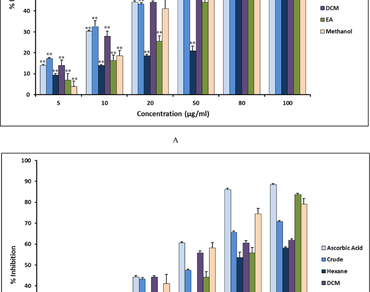
The aim of this study is to scientifically validate a novel Poly-Herbal Formulation (PHF) used by tribal communities of Upper Assam in treating respiratory diseases like pneumonia. The PHF is constituted by distinctive parts of four plants, barks of Azadirachta indica, seeds of Caesalpinia bonduc, roots of Solanum spirale and rhizomes of Cyperus rotundus grinded together in the ratio 1: 0.3: 1: 1. The different solvent extracts exhibited IC50 values in the range of 49.58–84.78 µg/ml (DPPH assay), 81.98–116.88 µg/ml (Nitric Oxide Scavenging Assay) and 4.023–8.243 mg/ml (Total Anti-oxidant assay) while the Zone of Inhibition (ZOI) for E. coli, P. aeruginosa and C. albicans measured within the range of 15–31 mm. Minimum Inhibitory Concentration (MIC) of the methanolic extract measured 0.002 mg/ml for E. coli, 0.002 mg/ml for P. aeruginosa and 0.012 mg/ml for C. albicans. Phenolic and flavonoid content analysis of methanolic extract revealed values equaling 63 ± 0.002 mg Gallic Acid Equivalent per 100 mg plant extract for phenolic content and 9.7 mg quercetin equivalent per 100 mg plant extract for flavonoid content. The FTIR of methanolic extract revealed the presence of functional characteristic peaks of ester, amine, acid, alcohol, alkane, alkene and aromatic compounds. Furthermore the GC–MS analysis revealed the presence of 10 phyto-constituents while some like 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- possess therapeutic properties like anti-oxidant, anti-microbial and anti-proliferative. Thus the PHF is a natural source of anti-oxidants and anti-microbial bioactive compounds.
References
Aeganathan R, Rayar A, Ilayaraja S, Prabakaran K, Manivannan R (2015) Anti-oxidant, anti-microbial evaluation and GC-MS analysis of Cyperus rotundus L. rhizomes chloroform fraction. Amer J Ethnomed 15:14–20
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16. https://doi.org/10.1093/jac/48.suppl_1.5
Arsul VA, Wagh SR, Mayee RV (2011) Hepatoprotective activity of livergen, a polyherbal formulation against carbon tetrachloride induced hepatotoxicity in rats. Int J Pharm Pharm Sci 3:228–231
Baba SA, Malik SA (2015) Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci 9:449–454. https://doi.org/10.1016/j.jtusci.2014.11.001
Cook NC, Samman S (1996) Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76. https://doi.org/10.1016/S0955-2863(95)00168-9
Cos P, Vlietinck AJ, Berghe DV, Maes L (2006) Anti-infective potential of natural products: how to develop a stronger in vitro “proof-of-concept.” J Ethnopharmacol 106:290–302. https://doi.org/10.1016/j.jep.2006.04.003
Curtis White W, Bellfield R, Ellis J, Vandendaele IP (2010) Controlling the spread of infections in hospital wards by the use of antimicrobials on medical textiles and surfaces. In: Anand SC, Kennedy JF, Miraftab M, Rajendran S (eds) Medical and healthcare textiles. Woodhead Publishing, pp 55–75
Das L, Das M, Barkalita LM, Borah P (2024) Comprehensive analysis of antioxidant properties, GC-MS, and FTIR profiles of Myrica esculenta fruit extracts from Western East Khasi Hills of Meghalaya. Chem Biodivers 21:e202401006. https://doi.org/10.1002/cbdv.202401006
Dutta A, Kakati R, Deka N, Hussain A, Ojah R, Borah D, Das M (2025) Phyto-chemical profiling, anti-oxidant and anti-microbial potential evaluation of the hydro-methanolic leaf extract of Aristolochia assamica, a new endemic species reported from NE India. Vegetos. https://doi.org/10.1007/s42535-025-01419-3
Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR (2008) Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol 7:3188–3192
Garg R, Perveen S, Gupta B, Bajpai VK (2015) Evaluation of antibacterial activity of Annona squamosa, Psidium guajava and Azadirachta indica against pathogenic bacterial cultures. Int J Curr Res Biosci Plant Biol 2:54–58
Ghayas S, Hannan A, Rizwani GH (2022) Phytochemical, antioxidant, toxicological, and pharmaceutical evaluation of polyherbal formulation: Irochel’. Dose Response 20:1–17. https://doi.org/10.1177/15593258211073412
Ghimeray AK, Jin C, Ghimire BK, Cho DH (2009) Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A. Juss grown in foothills of Nepal. Afr J Biotechnol 8:3084–3091
Harshitha D, Rodda R, Rao UMM (2013) Evaluation of polyherbal formulation, Livomyn for it’s hepatoprotective and antioxidant activity. Pharm Lett 5:135–141
Hazra B, Biswas S, Mandal N (2008) Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med 8:63. https://doi.org/10.1186/1472-6882-8-63
Huie RE, Padmaja S (1993) The reaction of no with superoxide. Free Radic Res Commun 18:195–199. https://doi.org/10.3109/10715769309145868
Jana K, Chatterjee K, Ali KM, Ghosh A, Bera TK, Ghosh D (2011) Antioxidant potential of hydro-methanolic extract of seed of Caesalpinia bonduc: an in vitro study. J Adv Pharm Technol Res 2:260–265. https://doi.org/10.4103/2231-4040.90884
Kalyana Sundaram I, Sarangi DD, Sundararajan V, George S, Sheik Mohideen S (2018) Poly herbal formulation with anti-elastase and anti-oxidant properties for skin anti-aging. BMC Complement Altern Med 18:33. https://doi.org/10.1186/s12906-018-2097-9
Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB (2019) Preliminary analysis of phytoconstituents and evaluation of anthelminthic property of Cayratia auriculata (in vitro). Maedica (Bucur) 14:350–356. https://doi.org/10.26574/maedica.2019.14.4.350
Keawsa-ard S, Liawruangrath B, Liawruangrath S, Teerawutgulrag A, Pyne SG (2012) Chemical constituents and antioxidant and biological activities of the essential oil from leaves of Solanum spirale. Nat Prod Commun 7:955–958
Keawsa-ard S, Liawruangrath B, Liaewruangrath S, Teerawutgulrag A, Pyne S (2016) Anticancer and antibacterial activities of the isolated compounds from Solanum spirale Roxb. leaves. Chiang Mai J Sci 43:546–554
Koleva II, van Beek TA, Linssen JP, de Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17. https://doi.org/10.1002/pca.611
Manorenjitha MS, Norita AK, Norhisham S, Asmawi MZ (2013) GC-MS analysis of bioactive components of Ficus religiosa (linn.) stem. Int J Pharm Bio Sci 4:99–103
Mythili K, Reddy UC, Chamundeeswari D, Manna PK, Mythili K (2013) GC-MS analysis of phytocomponents and in-vitro inhibitory effects of Calanthe triplicata. J Nat Prod(India) 6:141–146
Ohalete CN, Anyanwu GO (2023) Antimicrobial and phytochemical properties of neem leaf and bark extracts on selected microorganisms. J Agric Food Sci 21:194–207
Pal DK, Dutta S (2006) Evaluation of the antioxidant activity of the roots and rhizomes of Cyperus rotundus L. Indian J Pharm Sci 68:256–258. https://doi.org/10.4103/0250-474X.25731
Parasuraman S, Thing GS, Dhanaraj SA (2014) Polyherbal formulation: concept of ayurveda. Pharmacogn Rev 8:73–80. https://doi.org/10.4103/0973-7847.134229
Parekh J, Chanda S (2007) In vitro antimicrobial activity of Trapa natans L. fruit rind extracted in different solvents. Afr J Biotechnol 6:760–770
Payum T, Das A, Shankar R, Tamuly C, Hazarika M (2015) Antioxidant potential of Solanum spirale shoot and berry: a medicinal food plant used in Arunachal Pradesh. Int J PharmTech Res 5:307–314
Phongpaichit S, Nikom J, Rungjindamai N, Sakayaroj J, Hutadilok-Towatana N, Rukachaisirikul V, Kirtikara K (2007) Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol Med Microbiol 51:517–525. https://doi.org/10.1111/j.1574-695X.2007.00331.x
Ramamoorthy R, Muthalagu M, Andra S, Ravichandran B, Narayanasamy M (2019) Investigation on antimicrobial, antioxidant and cytotoxicity properties of triple bark extract formulated using traditional medicinal plants. SN Appl Sci 1:772. https://doi.org/10.1007/s42452-019-0791-y
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
Revathi P, Jeyaseelansenthinath T, Thirumalaikolundhusubramaian P (2014) Preliminary phytochemical screening and gc-ms analysis of ethanolic extract of mangrove plant-bruguiera cylindrica (rhizho) L. Int J Pharm Phyt Res 6:729–740
Sachan NK, Verma S, Sachan AK, Arshad H (2010) An investigation to antioxidant activity of Caesalpinia bonducella seeds. Ann Pharmacol Pharm Sci 1:88–91
Saeed MA, Sabir AW (2001) Antibacterial activity of Caesalpinia bonducella seeds. Fitoterapia 72:807–809. https://doi.org/10.1016/s0367-326x(01)00292-1
Senguttuvan J, Paulsamy S, Karthika K (2014) Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac J Trop Biomed 4:359–367. https://doi.org/10.12980/APJTB.4.2014C1030
Shanmugasundaram D, Duraiswamy A, Viswanathan A, Sasikumar CS, Cherian SM, Cherian KM (2016) Development of an antidiabetic polyherbal formulation (ADPHF6) and assessment of its antioxidant activity against ROS-induced damage in pUC19 and human lymphocytes - an in vitro study. J Complement Integr Med 13:267–274. https://doi.org/10.1515/jcim-2015-0028
Sharma SK, Singh AP (2011) Antimicrobial investigations on rhizomes of Cyperus rotundus Linn. Der Pharm Lett 3:427–431
Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP (2009) Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol 47:1848–1851. https://doi.org/10.1016/j.fct.2009.04.040
Singleton VL, Orthofer R, Lamuela Raventos RM (1990) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Packer L (ed) Methods in enzymology. Academic Press, pp 152–178
Srivastava S, Lal VK, Pant KK (2012) Polyherbal formulations based on Indian medicinal plants as antidiabetic phytotherapeutics. Phytopharm 2:1–15
Swarupa Rani G, R. Gunda R, Y. Mansa Y, Khushi Vardhan CH, Kumar VD, Venkateswarlu G (2015) Preparation and evaluation of antimicrobial and antioxidant activity of polyherbal ointment. Int J Curr Trends Pharm Res 3:997–1003
Tayung K, Sarkar M, Baruah P (2012) Endophytic fungi occurring in Ipomoea carnea tissues and their antimicrobial potentials. Braz Arch Biol Technol 55:653–660. https://doi.org/10.1590/S1516-89132012000500003
Ugochukwu SC, Uche AI, Ifeanyi O (2013) Preliminary phytochemical screening of different solvent extracts of stems bark and roots of Dennetia tripetala. Asian J Plant Sci 3:10–13
Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V (2000) Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba l.), and black tea (Camellia sinensis) extracts. J Agric Food Chem 48:5030–5034. https://doi.org/10.1021/jf000590k
Yu X, Zhao M, Liu F, Zeng S, Hu J (2013) Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products. J Food Res Int 51:397–403. https://doi.org/10.1016/j.foodres.2012.12.044
Author Information
Gauhati University, Guwahati, India