Biological activities of native Trichoderma species and their metabolites against Sclerotinia sclerotiorum
*Article not assigned to an issue yet
Research Articles | Published: 11 December, 2025
First Page: 0
Last Page: 0
Views: 378
Keywords: n Trichoderman , n Sclerotinia sclerotiorumn , Biocontrol, Secondary metabolites, Growth enhancement activity
Abstract
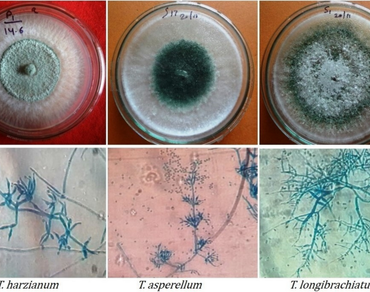
Present study aimed to evaluate the biological activities (antagonistic and plant growth promotion) of different species of Trichoderma and their crude metabolites against Sclerotinia sclerotiorum. Firstly, characterization of eight native isolates of Trichoderma species based on morphological and molecular (ITS-based) was done and among them 3 isolates of T. harzianum, 3 isolates of T. longibrachiatum and 2 isolates of T. asperellum were identified. The antagonistic potential of all eight Trichoderma isolates was evaluated by dual culture method, and a gliotoxin-producing isolate, T. asperellum T-21 was found the most effective to inhibit the growth of S. sclerotiorum by 92.7%. Volatile metabolic profile using gas chromatography mass spectrometry (GC–MS) was done and GC–MS analysis of ethyl acetate extract identified 28 volatile metabolites classified as fatty acids, fatty alcohols, ketones, esters, sesquiterpenoid, pyrazinoindole, and phenolic compounds. The antifungal assay of the crude extract from T. asperellum T-21 showed maximum inhibition efficiency at lower concentration (2.5 mg/mL), inhibiting S. sclerotiorum growth by 92.65%, while complete growth suppression was observed at 15 mg/mL. In planta study showed that extract of all isolates promotes plant growth parameters including germination percentage, root and shoot length, fresh weight, leaf area and chlorophyll content compared to untreated control. The crude extract from Trichoderma isolates at 3 mg/mL showed better growth enhancement activity than at 5 mg/mL concentration. In conclusion, isolate T. asperellum T-21 exhibited strong antagonistic potential against stem rot disease and demonstrated superior plant growth promotion activity compared to the other Trichoderma isolates.
References
Arnon D (1949) Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Asad SA (2022) Mechanisms of action and biocontrol potential of Trichoderma against fungal plant disease- a review. Ecol Complex 49:1000978
Ayaz M, Li C-H, Ali Q, Zhao W, Chi Y-K, Shafiq M, Ali F, Yu X-Y, Yu Q, Zhao J-T, Yu J-W, Qi R-D, Huang W-K (2023) Bacterial and fungal biocontrol agents for plant disease protection: journey from lab to field, current status, challenges, and global perspectives. Molecules 28(18):6735
Baazeem A, Almanea A, Manikandan P, Alorabi M, Vijayaraghavan P, Abdel-Hadi A (2021) In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J Fungi 7:331
Bano A, Qadri TA, Mahnoor KN (2023) Bioactive metabolites of plants and microbes and their role in agricultural sustainability and mitigation of plant stress. S Afr J Bot 159:98–109
Bissett J (1991a) A revision of the genus Trichoderma. II. Infrageneric classification. Can J Bot 69:2357–2372
Bissett J (1991b) A revision of the genus Trichoderma. III. Section Pachybasium. Can J Bot 69:2373–2417
Chaudhary S, Lal M, Sagar S, Sharma S, Meena AL, Kumar M (2023) Variation in oxalic acid production, mycelial compatibility and pathogenicity amongst isolates of Sclerotinia sclerotiorum causing white mold disease. Vegetos 37:1294–1306
Choez-Guaranda I, Espinoza-Lozano F, Reyes-Araujo D, Romero C, Manzano P, Galarza L, Sosa D (2023) Chemical characterization of Trichoderma spp. extracts with antifungal activity against Cocoa pathogens. Molecules 28(7):3208
De Jaeger N, de la Providencia IE, de Boulois HD, Declerck S (2011) Trichoderma harzianum might impact phosphorus transport by arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 77:558–567
Dennis C, Webster J (1971a) Antagonistic properties of species-groups of Trichoderma. I. Production of non-volatile antibiotics. Trans Br Mycol Soc 57:25–39
Dennis C, Webster J (1971b) Antagonistic properties of species-groups of Trichoderma. II. Production of volatile antibiotics. Trans Br Mycol Soc 57:41–48
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Druzhinina IS, Kopchinskiy AG, Kubicek CP (2006) The first one hundred of Trichoderma species is characterized by molecular data. Mycoscience 47:55–64
Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A et al (2011) Trichoderma: the genomics of opportunistic success. Nat Rev Microbiol 9:749–759
Duncan RW, Dilantha-Fernandoa WG, Rashid KY (2006) Time and burial depth influencing the viability and bacterial colonization of Sclerotinia sclerotiorum. Soil Biol Biochem 38:275–284
Fahmi AI, Eissa RA, El-Halfawi KA (2016) Identification of Trichoderma spp. by DNA barcode and screening for cellulolytic activity. J Microb Biochem Technol 8(3):202–209
Gams W, Bissett J (1998) Morphology and identification of Trichoderma. In: Harman GE, Kubicek CP (eds) Trichoderma and Gliocladium: basic biology, taxonomy and Genetics. Taylor & Francis Group
Guzmán-Guzmán P, Etesami H, Santoyo G (2025) Trichoderma: a multifunctional agent in plant health and microbiome interactions. BMC Microbiol 25:434
Haque Z, Iqbal M, Ahmad A, Khan MS, Prakash J (2020) Molecular characterization of Trichoderma spp. isolates by Internal Transcribed Spacer (ITS) region sequencing technique and its use as a biocontrol agent. Open Biotechnol J 14:70–77
Hu Z, Tao Y, Tao X, Su Q, Cai J, Qin C, Ding W, Li C (2019) Sesquiterpenes with phytopathogenic fungi inhibitory activities from Fungus Trichoderma virens from Litchi Chinensis Sonn. J Agric Food Chem 67:10646–10652
Hua L, Zeng H, He L, Jiang Q, Ye P, Liu Y, Sun X, Zhang M (2021) Gliotoxin is an important secondary metabolite involved in suppression of Sclerotium rolfsii of Trichoderma virens T23. Phytopathology 111(10):1720–1725
Hugar A, Nayaka S (2025) Trichoderma harzianum isolate AKH-5 enhances defense response in Cajanus cajan (L.) millsp. against Fusarium oxysporum f. sp. udum and promotes plant growth. The Microbe 8:100454
Jakobsen I, Murmann LM, Rosendahl S (2021) Hormetic responses in arbuscular mycorrhizal fungi. Soil Biol Biochem 159:108299
Jambhulkar PP, Singh B, Raja M, Ismaiel A, Lakshman DK, Tomar M, Sharma P (2024) Genetic diversity and antagonistic properties of Trichoderma strains from the crop rhizospheres in southern Rajasthan, India. Sci Rep 14:8610
Jayalakshmi R, Oviya R, Premalatha K et al (2021) Production, stability and degradation of Trichoderma gliotoxin in growth medium, irrigation water and agricultural soil. Sci Rep 11:16536
Johnson LF, Curl EA (1972) Methods for research on the soil borne plant pathogens. Burgess Pub, Minneapolis U.S., p 247
Kauserud H (2023) ITS alchemy: on the use of ITS as a DNA marker in fungal ecology. Fungal Ecol 65:101274
Khan RAA, Najeeb S, Hussain S, Xie B, Li Y (2020) Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 8(6):817
Kumar V, Jain L, Chaudhary S, Soni R (2022a) Endophytes as potential plant growth promoters in Forestry: Recent advances and perspectives. In: Molina G, Usmani Z, Sharma M, Yasri A, Gupta VK (eds) Microbes in agri-forestry biotechnology, 1st edn. CRC Press, Taylor & Francis Group, pp 241–262
Kumar V, Srivastava A, Jain L, Chaudhary S, Kaushal P, Soni R (2022b) Harnessing the potential of genetically improved bioinoculants for sustainable agriculture: Recent advances and perspectives. In: Soni R, Yadav AN, Suyal DC, Goel R (eds) Trends of Applied Microbiology for Sustainable Economy, Academic Press, Elsevier Inc., pp 319–341
Lakhdari W, Benyahia I, Bouhenna MM et al (2023) Exploration and evaluation of secondary metabolites from Trichoderma harzianum: GC-MS analysis, phytochemical profiling, antifungal and antioxidant activity assessment. Molecules 28(13):5025
Lopes FAC, Steindorff AS, Geraldine AM et al (2012) Biochemical and metabolic profiles of Trichoderma strains isolated from common bean crops in the Brazilian Cerrado and potential antagonism against Sclerotinia sclerotiorum. Fungal Biol 116(7):815–824
Lopez-Bucio J, Pelagio-Flores R, Herrera-Estrella A (2015) Trichoderma as biostimulant: exploiting the multilevel properties of a plant beneficial fungus. Scientia Hort 196:109–123
Marks BB, Nogueira MA, Hungria M (2025) Microbial secondary metabolites and their use in achieving sustainable agriculture: present achievements and future challenges. Agronomy 15(6):1350
Morton DJ, Stroube WH (1955) Antagonistic and stimulating effects of soil micro-organism of Sclerotium. Phytopathology 45:417–420
Mulatu A, Megersa N, Tolcha T, Alemu T, Vetukuri RR (2022) Antifungal compounds, GC-MS analysis and toxicity assessment of methanolic extracts of Trichoderma species in an animal model. PLoS ONE 17(9):e0274062
Nain MZ, Khan RU, Singh VP, Sayyeda S (2023) In vitro evaluation of antifungal activity of different Trichoderma spp. and plant extract against Sclerotinia sclerotiorum (Lib.) de Bary causing stem rot of Mustard. Int J Plant Sci 35(15):40–47
Natsiopoulos D, Tziolias A, Lagogiannis I, Mantzoukas S, Eliopoulos PA (2022) Growth-promoting and protective effect of Trichoderma atrobrunneum and T. simmonsii on tomato against soil-borne fungal pathogens. Crops 2(3):202–217
Oviya R, Thiruvudainambi S, Ramamoorthy V, Thamizhvendan R, Vellaikumar S (2022) Gas chromatography mass spectroscopy (GC-MS) analysis of the antagonistic potential of Trichoderma hamatum against Fusarium oxysporum f. sp. cepae causing basal rot disease of onion. J Biol Control 36(1):17–30
Pandey P, Kumar R, Mishra P (2010) Studies on pathogenic behaviour and carpogenic germination of sclerotia of Sclerotinia sclerotiorum causing stem rot of Chickpea. J Mycol Pl Pathol 40(2):192–196
Patidar P, Prasad L, Sagar S, Sirohi A, Saharan MS, Dhillon MK, Singh VK, Bag TK (2024) Chemo-profiling of Purpureocillium lilacinum and Paecilomyces variotii isolates using GC-MS analysis, and evaluation of their metabolites against M. incognita. PLoS ONE 19(2):e0297925
Pinar O, Rodríguez-Couto S (2024) Biologically active secondary metabolites from white-rot fungi. Front Chem 12:1363354
Prabhu S, Poorniammal R, Dufossé L (2025) Microbial metabolites: a sustainable approach to combat plant pests. Metabolites 15(6):418
Prasad L, Sagar S, Chaudhary S, Kumar N, Tomar A (2012) Determination of biodiversity using ITS-PCR-CAPS markers for Trichoderma sp. from rhizospheric soil of different location in India. Vegetos 25:336–341
Prasad L, Chaudhary S, Sagar S, Tomar A (2016) Mycoparasitic capabilities of diverse native strain of Trichoderma spp. against Fusarium oxysporum. J Appl Nat Sci 8(2):769–776
Raut I, Badea-Doni M, Calin M, Oancea F, Vasilescu G, Sesan TE, Jecu L (2014) Effect of volatile and non-volatile metabolites from Trichoderma spp. against important phytopathogens. Rev Chim Bucharest 65:1285–1288
Samuels GJ (2006) Trichoderma: systematics, the sexual state, and ecology. Phytopathology 96(2):195–206
Samuels GJ, Ismaiel A, Bom MC, Respinis S, Petrini O (2010) Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102(4):944–966
Sharma SK, Prasad L (2018) Bioactivity of Trichoderma asperellum against Colletotrichum asianum and Sclerotinia sclerotiorum. Pestic Res J 30(2):251–255
Sharma P, Samkumar A, Rao M, Singh VV, Prasad L, Mishra DC, Bhattacharya R, Gupta NC (2018) Genetic diversity studies based on morphological variability, pathogenicity and molecular phylogeny of the Sclerotinia sclerotiorum population from Indian mustard (Brassica juncea). Front Microbiol 9:1169
Singh M, Avtar R, Kumar N et al (2022) Genetic analysis for resistance to Sclerotinia stem rot, yield and its component traits in Indian Mustard [Brassica juncea (L.) Czern & Coss.]. Plants 11:671
Stewart A, Hill R (2014) Application of Trichoderma in plant growth promotion. In: Gupta VK, Scmoll M, Herrera-Estrella A, Upadhyay RS, Druzhinina I, Tuohy MG (eds) Biotechnology and biology of Trichoderma. Elsevier, Amsterdam, pp 415–428
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101(30):11030–11035
Vergara DEM, Barboza-Garcia A, Perez-Cordero A (2022) Antifungal and growth activity of strains of Trichoderma spp. against the Avocado “tristeza” disease, Phytophthora cinnamomic. Egypt J Biol Pest Control 32:115
Verma P, Chaudhary S, Sagar S, Singh A, Singh M, Tomar A (2025) Morpho-molecular characterization of native Trichoderma spp. isolates and their biocontrol efficacy against Fusarium oxysporum. Vegetos. https://doi.org/10.1007/s42535-025-01168-3
Villavicencio-Vásquez M, Espinoza-Lozano F, Espinoza-Lozano L, Coronel-León J (2025) Biological control agents: mechanisms of action, selection, formulation and challenges in agriculture. Front Agron 7:1578915
Vinale F, Manganiello G, Nigro M et al (2014) A novel fungal metabolite with beneficial properties for agricultural applications. Molecules 19:9760–9772
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press Inc, New York, pp 315–322
Yao X, Guo H, Zhang K, Zhao M, Ruan J, Chen J (2023) Trichoderma and its role in biological control of plant fungal and nematode disease. Front Microbiol 14:1160551
Zhang F, Ge H, Zhang F, Guo N, Wang Y, Chen L, Ji X, Li C (2016) Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol Biochem 100:64–74
Author Information
Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi, India