Biological control of papaya aphid (Aphis gossypii Glover) using entomopathogenic fungi
Mukherjee Anirvan, Debnath Prankrishna, Ghosh Swapan Kumar, Medda Pradyot Kumar
Research Articles | Published: 06 November, 2019
First Page: 1
Last Page: 10
Views: 4436
Keywords: Vector control, Entomopathogenic fungi, Papaya Ring Spot Virus, LD50 , LT50
Abstract
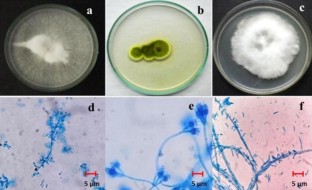
Papaya is economically important cultivated fruit crop grown in all tropical countries, having enormous nutritional values. Papaya Ring Spot Virus imposes a significant crop loss in terms of quality and quantity. To encounter the virus vector (Aphis gossypii), indiscriminate use of chemical pesticides creates severe environmental hazards whereas biological control is a perfect alternative to this problem. The objectives of our present study were isolation and characterization of indigenous fungi and their comparative analysis of entomopathogenic fungi against papaya aphid and finding its pathogenicity. Fungal isolates collected from natural sources were characterized and identified by the cultural and morphological study. Potential EPF genera were molecularly identified by PCR (ITS1-5.8S-ITS2) method. Entomopathogenic fungi were screened against A. gossypii for their pathogenecity by incised leaf disc method. LD50 (median lethal dose) and LT50 (median lethal time) were analyzed by regression analysis. Phylogenetic relationship among EPF was evaluated by MEGA software. Out of forty isolated entomopathogenic fungi, three (Beauveria bassiana deb4, Penicillium verrucosum Nlg1, and Fusarium equiseti khr4) were highly effective entomopathogen. The LD50 value of B. bassiana, P. verrucosum and F. equiseti were 1.4 × 104, 9.8 × 104, 1.0 × 106 spores ml−1, and LT50 values were 32.14, 37.5, 32.14 h respectively. Their phylogenetic analysis indicates related closeness on the basis of their conserved internal transcribed spacer region. In conclusion, the indigenous isolated strain of B. bassiana (deb4) has shown highest biocontrol potentiality amongst three indigenous entomopatogenic fungi under lab condition against A. gossypii and can be applied in agrifields.
References
- Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
- Binggeli O, Neyen C, Poidevin M, Lemaitre B (2014) Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog 10:e1004067
- Chakrabarti S, Raychaudhuri D (1978) New and little known aphids (Homoptera: Aphididae) from Kumaon Himalaya, India. Entomon 3(1):95–103
- Chutima R, Dell B, Vessabutr S, Bussaban B, Lumyong S (2011) Endophytic fungi from Pecteilis susannae (L.) Rafin (Orchidaceae), a threatened terrestrial orchid in Thailand. Mycorrhiza 21:221–229
- Dhingra OD, Sinclair JB (1985) Culture media and their formulas. Basic plant pathology methods. CRC, Boca Raton
- Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi, vol 1. Academic, London
- Fang W, Pava-Ripoll M, Wang S, Leger RS (2009) Protein kinase A regulates production of virulence determinants by the entomopathogenic fungus, Metarhizium anisopliae. Fung Genet Biol 46:277–285
- Freimoser FM, Screen S, Bagga S, Hu G, St. Leger RJ (2003) Expressed sequence tag (EST) analysis of two subspecies of Metarhizium anisopliae reveals a plethora of secreted proteins with potential activity in insect hosts. Microbiology 149:239–247
- Ganassi S, Moretti A, Stornelli C, Fratello B, Pagliai AB, Logrieco A, Sabatini MA (2001) Effect of Fusarium, Paecilomyces and Trichoderma formulations against aphid Schizaphis graminum. Mycopathologia 151:131–138
- Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
- Garkoti A, Kumar V, Tripathi H (2014) Control of wilt disease of lentil through bio control agents and organic amendments in Tarai region of Uttarakhand, India. J Environ Biol 35:1067–1070
- Ghosh SK, Chakraborty N, Biswas PP (2014) In vitro biological control of aphid of Papaya by Beauveria bassiana. III Int Symp Papaya 1022:113–117
- Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, Oxford
- Han JH, Jin BR, Kim JJ, Lee SY (2014a) Virulence of entomopathogenic fungi Metarhizium anisopliae and Paecilomyces fumosoroseus for the microbial control of Spodoptera exigua. Mycobiology 42:385–390
- Han Z, Wang Q, Fu J, Chen H, Zhao Y, Zhou B, Gong Z, Wei S, Li J, Liu H, Zhang X, Liu C, Yu H (2014b) Multiple bio-analytical methods to reveal possible molecular mechanisms of developmental toxicity in zebrafish embryos/larvae exposed to tris (2-butoxyethyl) phosphate. Aquat Toxicol 150:175–181
- Humber RA (2005) Fungal identification USDA-ARS plant protection research 103 Unit US plant. Soil & Nutrition Laboratory, Ithaca
- Jayasimha G, Rachana R, Rajkumar V, Manjunatha M (2013) Evaluation of fungal pathogen, Fusarium semitectum Berk and Ravenel against okra aphid, Aphis gossypii Glover under laboratory and green house conditions. Pest Manag Hort Ecosyst 18:139–142
- Kalleshwaraswamy CM, Krishnakumar NK, Chandrashekara KN, Vani A (2012) Efficacy of insecticides and oils on feeding behaviour of Aphis gossypii Glover and transmission of Papaya ringspot virus (PRSV). K J A S 25(1):63–67
- Karthikeyan A, Selvanarayanan V (2011) In vitro efficacy of Beauveria bassiana (Bals.) Vuill. and Verticillium lecanii (Zimm.) viegas against selected insect pests of cotton. Recent Res Sci Technol 3(2):142–143
- Kim JJ, Jeong G, Han JH, Lee S (2013) Biological control of aphid using fungal culture and culture filtrates of Beauveria bassiana. Mycobiology 41:221–224
- Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
- Lv C, Huang B, Qiao M, Wei J, Ding B (2011) Entomopathogenic fungi on Hemiberlesia pitysophila. PLoS One 6:e23649
- Martinuz A, Schouten A, Menjivar R, Sikora R (2012) Effectiveness of systemic resistance toward Aphis gossypii (Hom., Aphididae) as induced by combined applications of the endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Bio Control 62:206–212
- Mishra DS, Kumar A, Prajapati CR, Singh A, Sharma S (2013) Identification of compatible bacterial and fungal isolate and their effectiveness against plant disease. J Environ Biol 34:183
- Morozova VV, Gusakov AV, Andrianov RM, Pravilnikov AG, Osipov DO, Sinitsyn AP (2010) Cellulases of Penicillium verruculosum. Biotechnol J 5:871–880
- Nagamani A, Kunwar IK, Manoharachary C (2006) Hand book of soil fungi. IK International Pvt. Ltd., New Delhi, p 477
- Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, Oxford
- Ng JC, Perry KL (2004) Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5:505–511
- Nicoletti R, De Stefano S (2000) Peptidi ciclici di origine fungina. IL Tabacco 8:33–59
- Ogawa K, Miura T (2014) Aphid polyphenisms: trans-generational developmental regulation through viviparity. Front Physiol 5:1
- Ortiz-Urquiza A, Keyhani NO (2013) Action on the surface: entomopathogenic fungi versus the insect cuticle. Insects 4:357–374
- Patil NS, Jadhav JP (2015) Significance of Penicillium ochrochloron chitinase as a biocontrol agent against pest Helicoverpa armigera. Chemosphere 128:231–235
- Podder D, Ghosh SK (2019) A new application of Trichoderma asperellum as an anopheline larvicide for eco friendly management in medical science. Sci Rep 9:1108. https://doi.org/10.1038/s41598-018-37108-2
- Quesada-Moraga E, Carrasco-Díaz JA, Santiago-Álvarez C (2006) Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae). J Appl Entomol 130:442–452
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
- Shah HU, Iqbal Z (2017) Biological screening of crude extract of Penicillium sp. EU0013. J Anim Plant Sci 27(4):1209–1216
- Sharma P, Saini MK, Deep S, Kumar V (2012) Biological control of groundnut root rot in farmer’s field. J Agric Sci 4(8):48
- Tajima F (1993) Simple methods for testing the molecular evolutionary clock hypothesis. Genetics 135:599–607
- Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci 101:11030–11035
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
- Tennant PF, Fermin GA, Roye ME (2007) Viruses infecting papaya (Carica papaya L.): etiology, pathogenesis and molecular biology. Plant Viruses. 1:178–188
- Verma M, Brar SK, Tyagi RD, Surampalli RY, Valero JR (2007) Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng J 37:1–20
- White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genesfor phylogenetics. PCR Protoc 18:315–322
- Zimmermann G (1986) The ‘Galleria bait method for detection of entomopathogenic fungi in soil. J Appl Entomol 102:213–215
Author Information
Molecular Mycopathology Laboratory, Biocontrol and Cancer Research Unit, P.G. Department of Botany, Ramakrishna Mission Vivekananda Centenary College (Autonomous), Kolkata, India