Bioprospecting antioxidants in some non-heterocystous filamentous cyanobacteria inhabit water bodies of semi-arid Rajasthan in India
Research Articles | Published: 20 July, 2020
First Page: 601
Last Page: 609
Views: 4678
Keywords: Antioxidants, Cyanobacteria, Bioprospects, Phycobiliproteins, Pigments, Phenolics
Abstract
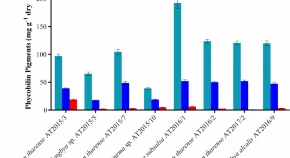
Cyanobacteria are ancient photosynthetic organisms inhabitant of various habitats including arid and semi- arid water bodies. These are effective producers of various biologically active compounds that have been widely used in food, medicine, cosmetics and pharmaceutical industry. However, non-heterocystous cyanobacteria of arid and semi- arid water bodies are less explored for biotechnological applications. The aim of the present study was to investigate non-heterocystous filamentous cyanobacteria of freshwater bodies of semi-arid region for their pigment profile, phenolic contents and antioxidant properties. Our results revealed that among 8 cyanobacterial strains, D. tharense AT2015/7 and S. subsalsa AT2016/1 possessed higher amount of valuable pigments, phenolic contents and also showed good antioxidant properties. S. subsalsa AT2016/1 was the most promising cyanobacterial taxa for the production of phycocyanin when compared to so far studied non-heterocystous cyanobacteria inhabit aquatic habitats. In the LC-HRMS/MS analysis, 11 phenolic compounds were tentatively identified in D. tharense AT2015/7 and nine phenolic compounds detected in S. subsalsa AT2016/1. The present study revealed that cyanobacteria thriving in the semi-arid region could be promising candidates for the production of valuable pigments, phenolic compounds and could be a potential source of antioxidants.
References
- Agyei D, Danquah MK, Sarethy IP, Pan S (2015) Antioxidative peptides derived from food proteins. In: Rani V, Yadav U (eds) Free Radicals in human health and disease. Springer, New Delhi, pp 417–430
- Aiba S, Ogawa T (1977) Assessment of growth yield of a blue–green alga, Spirulina platensis, in axenic and continuous culture. J Gen Microbiol 102(1):179–182
- Amchova P, Kotolova H, Ruda-Kucerova J (2015) Health safety issues of synthetic food colorants. Regul Toxicol Pharmacol 73(3):914–922
- de Araújo RFF, Martins DBG, Borba MACSM (2016) Oxidative stress and disease. In: Morales-Gonzalez JA, Madrigal-Santillan EO (eds) A master regulator of oxidative stress—the transcription factor Nrf2. IntechOpen, London
- Babić O, Kovač D, Rašeta M et al (2016) Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. J Appl Phycol 28(4):2333–2342
- Badr OA, El-Shawaf II, El-Garhy HA et al (2019) Antioxidant activity and phycoremediation ability of four cyanobacterial isolates obtained from a stressed aquatic system. Mol Phylogenet Evol 134:300–310
- Barbosa JEDL, Medeiros ESF, Brasil J et al (2012) Aquatic systems in semi-arid Brazil: limnology and management. Acta Limnol Bras 24(1):103–118
- Belay A (2013) Biology and industrial production of Arthrospira (Spirulina). In: Richmond A, HU Q (eds) Handbook of Microalgal Culture: Applied Phycology and Biotechnology: Second Edition. Blackwell, Oxford, pp 339–358
- Bennett A, Bogobad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58(2):419–435
- Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
- Blagojević D, Babić O, Rašeta M et al (2018) Antioxidant activity and phenolic profile in filamentous cyanobacteria: the impact of nitrogen. J Appl Phycol 30(4):2337–2346
- Brendonck L, Williams WD (2000) Biodiversity in wetlanands of dry regions (Drylands). In: Gopal B, Junk WJ, Davis JA (eds) Biodiveristy in wetlands: Assessment, function and conservation. Backhuys, Leiden, pp 181–194
- Clark RL, McGinley LL, Purdy HM et al (2018) Light-optimized growth of cyanobacterial cultures: growth phases and productivity of biomass and secreted molecules in light-limited batch growth. Metab Eng 47:230–242
- Dadheech PK, Abed RMM, Mahmoud H et al (2012a) Polyphasic characterization of cyanobacteria isolated from desert crusts, and the description of Desertifilum tharense gen. et sp. nov. (Oscillatoriales). Phycologia 51(3):260–270
- Dadheech PK, Mahmoud H, Kotut K, Krienitz L (2012b) Haloleptolyngbya alcalis gen. et sp. nov., a new filamentous cyanobacterium from the soda lake Nakuru, Kenya. Hydrobiologia 691(1):269–283
- Dasgupta CN (2016) Algae as a source of phycocyanin and other industrially important pigments. In: Das D (ed) Algal biorefinery: an integrated approach. Springer, Cham, pp 253–276
- de Castro ML, Mattos A, Lürling M, Becker V (2015) Is the future blue-green or brown? The effects of extreme events on phytoplankton dynamics in a semi-arid man-made lake. Aquat Ecol 49(3):293–307
- Espín JC, Soler-Rivas C, Wichers HJ (2000) Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem 48(3):648–656
- Gantar M, Simović D, Djilas S et al (2012) Isolation, characterization and antioxidative activity of C-phycocyanin from Limnothrix sp. strain 37-2-1. J Biotechnol 159(1–2):21–26
- Gopal B (2003) Aquatic biodiversity in arid and semi-arid zones of Asia and water management. In: Lemons J, Victor R, Schaffer D (eds) Conserving Biodiversity in Arid Regions. Springer, Boston, pp 199–215
- Hajimahmoodi M, Faramarzi MA, Mohammadi N et al (2010) Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol 22(1):43–50
- Havens KE, James RT, East TL, Smith VH (2003) N: P ratios, light limitation, and cyanobacterial dominance in a subtropical lake impacted by non-point source nutrient pollution. Environ Pollut 122(3):379–390
- Heidari F, Zima J, Riahi H, Hauer T (2018) New simple trichal cyanobacterial taxa isolated from radioactive thermal springs. Fottea 18(2):137–149
- Hossain MF, Ratnayake RR, Meerajini K, Wasantha Kumara KL (2016) Antioxidant properties in some selected cyanobacteria isolated from fresh water bodies of Sri Lanka. Food Sci Nutr 4(5):753–758
- Kepekçi RA, Saygideger SD (2012) Enhancement of phenolic compound production in Spirulina platensis by two-step batch mode cultivation. J Appl Phycol 24(4):897–905
- King JL, Simovich MA, Brusca RC (1996) Species richness, endemism and ecology of crustacean assemblages in Northern California vernal pools. Hydrobiologia 328(2):85–116
- Komárek J, Anagnostidis K (2005) Süßwasserflora von Mitteleuropa, bd. 19/2: Cyanoprokaryota: Oscillatoriales. Spektrum Akademischer Verlag, München
- Kumar J, Parihar P, Singh R et al (2016) UV-B induces biomass production and nonenzymatic antioxidant compounds in three cyanobacteria. J Appl Phycol 28(1):131–140
- Loza V, Perona E, Mateo P (2014) Specific responses to nitrogen and phosphorus enrichment in cyanobacteria: factors influencing changes in species dominance along eutrophic gradients. Water Res 48(1):622–631
- Machu L, Misurcova L, Ambrozova JV et al (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20(1):1118–1133
- Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreira WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97(18):2402–2406
- Nunnery JK, Mevers E, Gerwick WH (2010) Biologically active secondary metabolites from marine cyanobacteria. Curr Opin Biotechnol 21:787–793
- Pagels F, Guedes AC, Amaro HM et al (2019) Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnol Adv 37:422–443
- Pan-utai W, Iamtham S (2019) Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem 82:189–198
- Patel VK, Sundaram S, Patel AK, Kalra A (2018) Characterization of seven species of cyanobacteria for high-quality biomass production. Arab J Sci Eng 43(1):109–121
- Prakash JW, Johnson M, Jeeva S (2011) Antimicrobial activity of certain fresh water microalgae from Thamirabarani River, Tamil Nadu, South India. Asian Pac J Trop Biomed 1(2):S170–S173
- Rippka R, Deruelles J, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111(1):1–61
- Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
- Sies H (1997) Physiological society symposium: Impaired endothelial and smooth muscle cell function in oxidative stress. Exp Physiol 82:291–295
- Silva HJ, Italiano MC, Ferrari SG (1994) Improved biomass production of cyanobacteria by reutilization of the culture medium. Biotechnol Tech 8(12):889–894
- Singh DP, Prabha R, Verma S et al (2017) Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech 7(2):134
- Stanley EH, Fisher SG (1992) Aquatic ecosystems in semi-arid regions: implications for resource management. In: N.H.R.I. Symposium Series 7, Environment Canada. The Institute, pp 271–280
- Tomer AK, Neelam DK, Dadheech PK (2018) Pigments profiling of non-heterocystous filamentous cyanobacterial taxa (oscillatoriales) inhabited in biological crusts and soda lake. Vegetos 31(1):43–50
- Vaz JA, Heleno SA, Martins A et al (2010) Wild mushrooms Clitocybe alexandri and Lepista inversa: in vitro antioxidant activity and growth inhibition of human tumour cell lines. Food Chem Toxicol 48(10):2881–2884
- Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313
- Witham CW, Bauder ET, Belk D, et al (1998) Ecology, conservation, and management of vernal pool ecosystems: proceedings from the 1996 california native plant society conference. California Native Plant Society
Author Information
Department of Microbiology, School of Life Sciences, Central University of Rajasthan, Ajmer, India