Changes in vegetation cover and soil intrinsic properties influence the soil bacterial community composition and diversity across different climatic regions of India
Dinakaran J., Vikram Krati, Hanief Mohd, Bidalia Ankita, Tambat Subodh, Rao K. S.
Research Articles | Published: 27 May, 2019
First Page: 288
Last Page: 302
Views: 4240
Keywords: Soil organic carbon, Soil nitrogen, Illumina sequencing, Soil bacterial diversity, Climatic regions
Abstract
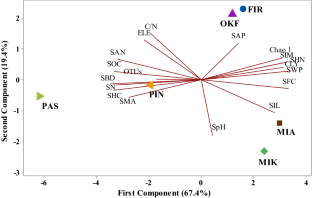
Soil microbial community in forest ecosystems plays a significant role in carbon and nutrient cycling. Very little is known about the effect of vegetation cover on soil bacterial community composition and diversity across different climatic regions of India. Soil was sampled from the plant cover dominated by seasonal herbs and grasses (PAS), Pinus roxburghii Sarg (PIN), Abies pindrow L. (FIR), Quercus incana Roxb (OKF), Mitragyna parvifolia Roxb (MIK); Acacia nilotica L. and Salvadora spp. (MIA) in three different climatic regions (humid, moist sub-humid and semi-arid) of India. The soil physical, chemical and biological properties such as sand (SAN), silt (SIL), clay (CLY), bulk density (SBD), wilting point (SWP), field capacity (SFC), saturated hydraulic conductivity (SHC), pH (SpH), organic carbon (SOC), nitrogen (SN), C:N ratio, available phosphorous (SAP) and total microbial activity (SMA) were determined. Illumina sequencing of specific 16S rRNA gene was applied to identify bacterial community composition in the soils under different vegetation cover. Results showed that the soil properties varied under different vegetation cover across the different climatic regions. SOC, SN, SMA were highest in the moist sub-humid region sites (PAS, PIN) followed by humid region (OKF, FIR) and semi-arid region (MIK, MIA) sites. However, the Chao 1 (species richness), Shannon and Simpson index (diversity) were highest in OKF, followed by MIK, FIR, MIA, PIN and PAS. The predominant bacterial phyla and genera in the soils under different vegetation cover were Proteobacteria, Actinobacteria, Bacteroidetes, Firmicutes, Planctomycetes, Verrucomicrobia, Thermotogae and Geobacter, Methylocapsa, Sphingomonas, Pseudomonas, Erysipelotrichaceae_incertae_sedis, Sporotomaculum, Amorphus, Helicobacter, Paenibacillus, Bauldia, Skermanella, Methylosinus, Singulisphaera, Marinobacter and Lamprocystis. We also found the exclusive OTUs abundance of some bacterial phyla and genera in the soils, which were not correlated with any one of the studied soil variables. In our analysis, we found only the Firmicutes, Verrucomicrobia and Bacteroidetes are linearly correlated (P < 0.05) with CLY, SWP and SpH. Likewise, bacterial genera Methylocapsa, Methylosinus and Amorphus were linearly correlated (P < 0.05) with SIL, SpH, C:N ratio and SAP. Our results suggested that the type of vegetation cover has a significant impact on changes in soil properties, controlling the soil bacterial community composition and diversity across different climatic regions of India. The soil bacterial community composition and diversity may serve as a potential ecological indicator with respect to land use and land cover change on biogeochemical cycling processes across different climatic regions of India.
References
- Adam G, Duncan H (2001) Development of sensitive and rapid method for the measurement of total microbial activity using fluroscein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
- Alexander L (2016) Global observed long-term changes in temperature and precipitation extremes: a review of progress and limitations in IPCC assessments and beyond. Weather Clim Extrem 11:4–16
- Allen SE, Grimshaw H, Parkinson JA, Quarmby CL (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications, Hoboken
- Arndt D, Xia J, Liu Y, Zhou Y, Guo A, Cruz J, Sinelnikov I, Budwill K, Nesbo C, Wishart D (2012) METAGENassist: a comprehensive web server for comparative metagenomics. Nucl Acids Res 40:W88–W95
- Baubin C, Farrell A, Stovicek A, Ghazaryan L, Giladi I, Gillor O (2019) Seasonal and spatial variability in total and active bacterial communities from desert soil. Pedobiologia 74:7–14
- Berg A, Lintner B, Findell K, Seneviratne S, van den Hurk B, Ducharne A, Chéruy F, Hagemann S, Lawrence D, Malyshev S, Meier A, Gentine P (2015) Interannual coupling between summertime surface temperature and precipitation over land: processes and implications for climate change. J Clim 28:1308–1328
- Bergmann G, Bates S, Eilers K, Lauber C, Caporaso J, Walters W, Knight R, Fierer N (2011) The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol Biochem 43:1450–1455
- Berlemont R, Martiny A (2014) Genomic potential for polysaccharide deconstruction in bacteria. Appl Environ Microbiol 81:1513–1519
- Binkley D, Giardina C (1998) Why do trees affect soils in temperate and tropical forests? The warp and woof of tree/soil interactions. Biogeochemistry 42:89–106
- Bonan G (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320:1444–14449. https://doi.org/10.1126/science.1155121
- Cai Y, Zheng Y, Bodelier P, Conrad R, Jia Z (2016) Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat Commun 7:11728. https://doi.org/10.1038/ncomms11728
- Canadell J, Schulze E (2014) Global potential of biospheric carbon management for climate mitigation. Nat Commun 5:6. https://doi.org/10.1038/ncomms6282
- Chaturvedi R, Gopalakrishnan R, Jayaraman M, Bala G, Joshi N, Sukumar R, Ravindranath N (2010) Impact of climate change on Indian forests: a dynamic vegetation modelling approach. Mitig Adapt Strat Gl 16:119–142
- Chodak M, Klimek B, Azarbad H, Jaźwa M (2015) Functional diversity of soil microbial communities under Scots pine, Norway spruce, silver birch and mixed boreal forests. Pedobiologia 58:81–88
- Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples, version 9. User’s guide and application. http://purl.oclc.org/estimates
- Conrad R (2007) Microbial ecology of methanogens and methanotrophs. Adv Agron 96:1–63
- Delgado-Baquerizo M, Maestre F, Reich P, Jeffries T, Gaitan J, Encinar D, Berdugo M, Campbell C, Singh B (2016) Microbial diversity drives multi functionality in terrestrial ecosystems. Nat Commun 7:10541
- Dinakaran J, Chandra A, Chamoli KP, Deka J, Rao KS (2018) Soil organic carbon stabilization changes with an altitude gradient of land cover types in central Himalaya, India. CATENA 170:374–385
- Eaton WD, Mcdonald S, Roed M, Vandecar KL, Hauge JB, Barry D (2011) A comparison of nutrient dynamics and microbial community characteristics across seasons and soil types in two different old growth forests in Costa Rica. Trop Ecol 52:35–48
- Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998
- Faoro H, Alves A, Souza E, Rigo L, Cruz L, Al-Janabi S, Monteiro R, Baura V, Pedrosa F (2010) Influence of soil characteristics on the diversity of bacteria in the Southern Brazilian Atlantic Forest. Appl Environ Microbiol 76:4744–4749
- Fierer N, Bradford M, Jackson R (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
- Franciolia D, Ascher J, Ceccherini M, Pietramellara G (2014) Land use and seasonal effects on a Mediterranean soil bacterial community. J Soil Sci Plant Nut 14:710–722
- Gee GW, Bauder JW (1986) Particle-size Analysis. P. 383–411. In Page AL (ed.). Methods of soil analysis, Part1, Physical and mineralogical methods. Second Edition, Agronomy Monograph 9, American Society of Agronomy, Madison, WI
- Gill RA, Burke IC (1999) Ecosystem consequences of plant life form changes at three sites in the semiarid United States. Oecologia 121:551–563
- Gopalakrishnan R, Jayaraman M, Bala G, Ravindranath NH (2011) Climate change and Indian forests. Curr Sci 101:348–355
- Gougoulias C, Clark J, Shaw L (2014) The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric 94:2362–2371
- Grace J, Mitchard E, Gloor E (2014) Perturbations in the carbon budget of the tropics. Glob Chang Biol 20:3238–3255
- Hansel C, Fendorf S, Jardine P, Francis C (2008) Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl Environ Microbiol 74:1620–1633
- He J, Fang J, Wang Z, Guo D, Flynn D, Geng Z (2006) Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 149:115–122
- He X, Hou E, Liu Y, Wen D (2016) Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China. Sci Rep 6:24261. https://doi.org/10.1038/srep24261
- Huang W, Bai Z, Hoefel D, Hu Q, Lv X, Zhuang G, Xu S, Qi H, Zhang H (2012) Effects of cotton straw amendment on soil fertility and microbial communities. Fron Environ Sci En 6:336–349
- Janssen P (2006) Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA Genes. Appl Environ Microbiol 72:1719–1728
- Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–426
- Karimi B, Maron P, Chemidlin-Prevost Boure N, Bernard N, Gilbert D, Ranjard L (2017) Microbial diversity and ecological networks as indicators of environmental quality. Environ Chem Lett 15:265–281
- Klimek B, Niklińska M, Jaźwa M, Tarasek A, Tekielak I, Musielok Ł (2015) Covariation of soil bacteria functional diversity and vegetation diversity along an altitudinal climatic gradient in the Western Carpathians. Pedobiologia 58:105–112
- Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glockner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41(1):e1. https://doi.org/10.1093/nar/gks808
- Kuramae E, Yergeau E, Wong L, Pijl A, Veen J, Kowalchuk G (2011) Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbiol Ecol 79:12–24
- Lauber C, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil ph as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
- Leff J, Jones S, Prober S, Barberán A, Borer E, Firn J, Harpole W, Hobbie S, Hofmockel K, Knops J, McCulley R, La Pierre K, Risch A, Seabloom E, Schütz M, Steenbock C, Stevens C, Fierer N (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Nat Acad Sci USA 112:10967–10972
- Li N, Shao T, Zhu T, Long X, Gao X, Liu Z, Shao H, Rengel Z (2018) Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci Rep 8:9728. https://doi.org/10.1038/s41598-018-28054-0
- Lladó S, López-Mondéjar R, Baldrian P (2017) Forest soil bacteria: diversity, involvement in ecosystem processes, and response to global change. Microbiol Mol Biol Rev 81:e00063–e00016
- Loo Y, Billa L, Singh A (2015) Effect of climate change on seasonal monsoon in Asia and its impact on the variability of monsoon rainfall in Southeast Asia. Geosci Front 6:817–823
- Lutzow MV, Knabner IK, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, Marschner B (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39:2183–2207
- Louis B, Maron P, Menasseri-Aubry S, Sarr A, Lévêque J, Mathieu O, Jolivet C, Leterme P, Viaud V (2016) Microbial diversity indexes can explain soil carbon dynamics as a function of carbon source. PLoS One 11:e0161251
- Lüneberg K, Schneider D, Siebe C, Daniel R (2018) Drylands soil bacterial community is affected by land use change and different irrigation practices in the Mezquital Valley. Mexico. Sci Rep 8:1413. https://doi.org/10.1038/s41598-018-19743-x
- Maestre F, Delgado-Baquerizo M, Jeffries T, Eldridge D, Ochoa V, Gozalo B, Quero J, García-Gómez M, Gallardo A, Ulrich W, Bowker M, Arredondo T, Barraza-Zepeda C, Bran D, Florentino A, Gaitán J, Gutiérrez J, Huber-Sannwald E, Jankju M, Mau R, Miriti M, Naseri K, Ospina A, Stavi I, Wang D, Woods N, Yuan X, Zaady E, Singh B (2015) Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc Nat Acad Sci USA 112:15684–15689
- Marty C, Houle D, Gagnon C, Courchesne F (2017) The relationships of soil total nitrogen concentrations, pools and C: N ratios with climate, vegetation types and nitrate deposition in temperate and boreal forests of eastern Canada. CATENA 152:163–172
- Metherell AK, Harding LA, Cole CV, and Parton WJ (1993) CENTURY Soil organic matter model environment. Technical documentation. Agroecosystem version 4.0. Great Plains System Research Unit Technical Report No. 4. USDA-ARS, Fort Collins, Colorado, USA
- National bureau of soil survey (NBSS) Staff (1985) Soil Map of India (1:7 M). NBSS & LUP, Nagpur
- Navarrete A, Soares T, Rossetto R, van Veen J, Tsai S, Kuramae E (2015) Verrucomicrobial community structure and abundance as indicators for changes in chemical factors linked to soil fertility. Antonie Van Leeuwenhoek 108:741–752
- Neher D, Weicht T, Bates S, Leff J, Fierer N (2013) Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS One 8:e79512
- Nepstad DC, Carvalho CR, Davidson EA, Jipp PH, Letebvre PA, Negreiros GH, Silva ED, Stone TA, Trumbore SE, Vieira S (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:666–669
- Pan Y, Birdsey R, Fang J et al (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
- Patel V, Sharma A, Lal R, Al-Dhabi NA, Madamwar H (2016) Response and resilience of soil microbial communities inhabiting in edible oil stress/contamination from industrial estates. BMC Microbiol 16:50. https://doi.org/10.1186/s12866-016-0669-8
- Prakash O, Gihring T, Dalton D, Chin K, Green S, Akob D, Wanger G, Kostka J (2010) Geobacter daltonii sp. nov., an Fe(III)- and uranium(VI)-reducing bacterium isolated from a shallow subsurface exposed to mixed heavy metal and hydrocarbon contamination. Int J Syst Evol Microbiol 60:546–553
- Raju BMK, Rao KV, Venkateswarlu B, Rao AVMS, Rama Rao CA, Rao VUM, Bapuji Rao B, Ravi Kumar N, Dhakar R, Swapna N, Latha P (2013) Revisiting climatic classification in India: a district-level analysis. Curr Sci 105:492–495
- Ramirez K, Leff J, Barberan A, Bates S, Betley J, Crowther T, Kelly E, Oldfield E, Shaw E, Steenbock C, Bradford M, Wall D, Fierer N (2014) Biogeographic patterns in below-ground diversity in New York City’s Central Park are similar to those observed globally. Philos Trans R Soc Lond B Biol Sci 281:20141988
- Reed S, Cleveland C, Townsend A (2011) Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Syst 42:489–512
- Ren B, Hu Y, Chen B, Zhang Y, Thiele J, Shi R, Liu M, Bu R (2018) Soil pH and plant diversity shape soil bacterial community structure in the active layer across the latitudinal gradients in continuous permafrost region of Northeastern China. Sci Rep 8:5619. https://doi.org/10.1038/s41598-018-24040-8
- Rodrigues J, Pellizari V, Mueller R, Baek K, Jesus E, Paula F, Mirza B, Hamaoui G, Tsai S, Feigl B, Tiedje J, Bohannan B, Nusslein K (2012) Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc Nat Acad Sci USA 110:988–993
- Rousk J, Bååth E, Brookes P, Lauber C, Lozupone C, Caporaso J, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
- Russo S, Legge R, Weber K, Brodie E, Goldfarb K, Benson A, Tan S (2012) Bacterial community structure of contrasting soils underlying Bornean rain forests: inferences from microarray and next-generation sequencing methods. Soil Biol Biochem 55:48–59
- Schimel J, Schaeffer S (2012) Microbial control over carbon cycling in soil. Front Microbiol 3:1–11. https://doi.org/10.3389/fmicb.2012.00348
- Shukla P, Pandey K, Mishra V (2013) Environmental determinants of soil methane oxidation and methanotrophs. Crit Rev Environ Sci Technol 43:1945–2011
- Sundqvist M, Sanders N, Wardle D (2013) Community and ecosystem responses to elevational gradients: processes, mechanisms, and insights for global change. Annu Rev Ecol Syst 44:261–280
- ter Braak CJF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
- Tian Q, Taniguchi T, Shi W, Li G, Yamanaka N, Du S (2017) Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci Rep 7:45289. https://doi.org/10.1038/srep45289
- Tripathi B, Song W, Slik J, Sukri R, Jaafar S, Dong K, Adams J (2016) Distinctive tropical forest variants have unique soil microbial communities, but not always low microbial diversity. Front Microbiol 7:22–32
- Trivedi P, Anderson I, Singh B (2013) Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol 21(12):641–651
- Trivedi P, Delgado-Baquerizo M, Anderson I, Singh B (2016) Response of soil properties and microbial communities to agriculture: implications for primary productivity and soil health indicators. Front Plant Sci 7:1–13
- Trivedi P, Anderson I, Singh B (2018) Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol 21:641–651
- Truu M, Ostonen I, Preem J, Lõhmus K, Nõlvak H, Ligi T, Rosenvald K, Parts K, Kupper P, Truu J (2017) Elevated air humidity changes soil bacterial community structure in the silver birch stand. Front Microbiol 8:1–15. https://doi.org/10.3389/fmicb.2017.00557
- Uroz S, Calvaruso C, Turpault M, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol 17:378–387
- Viulu S, Nakamura K, Okada Y, Saitou S, Takamizawa K (2012) Geobacter luticola sp. nov., an Fe(III)-reducing bacterium isolated from lotus field mud. Int J Syst Evol Microbiol 63:442–448
- Wang R, Zhang H, Sun L, Qi G, Chen S, Zhao X (2017) Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-00472-6
- Zeng Q, Dong Y, An S (2016) Bacterial community responses to soils along a latitudinal and vegetation gradient on the loess plateau, China. PLoS One 11:e0152894
- Zhang X, Chen Q, Han X (2013) Soil bacterial communities respond to mowing and nutrient addition in a steppe ecosystem. PLoS One 8:e84210
- Zhang Y, Shen H, He X, Thomas B, Lupwayi N, Hao X, Thomas M, Shi X (2017) Fertilization shapes bacterial community structure by alteration of soil pH. Front Microbiol 8:1325. https://doi.org/10.3389/fmicb.2017.01325
- Zhou S, Yang G, Lu Q, Wu M (2014) Geobacter soli sp. Nov a dissimilatory Fe(III)-reducing bacterium isolated from forest soil. Int J Syst Evol Microbiol 64:3786–3791
Author Information
Natural Resource Management Laboratory, Department of Botany, University of Delhi (North Campus), Delhi, India