Characterization and biocontrol measures of Pseudomonas syringae pv. syringae associated with citrus blast disease
Research Articles | Published: 22 July, 2020
First Page: 555
Last Page: 569
Views: 4017
Keywords: Pseudomonas syringae , Molecular detection, syrB Gene amplification, Biological control, Antagonistic activity, In vivo management
Abstract
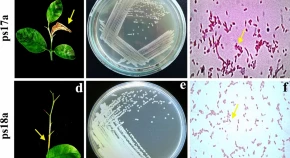
The present study was conducted to identify the two Pseudomonas syringae pv. syringae strains attained from the citrus blast infected tissues and also to find out the antimicrobial potentiality of plant extracts and antagonistic bacteria Bacillus spp. both under in vitro and in vivo conditions. Based on the microscopic observation and biochemical features including colouring, magnitude, size, and form of colonies displayed on LB medium, we speculate that ps17a and ps18a strains were similar to P. syringae pv syringae. For molecular identification, 16S rRNA and multi-locus sequence analysis (MLSA) with two housekeeping gene (rpoD and gyrB) sequences indicated an identical pattern to the corresponding sequences of other P. syringae pv. syringae strains which confirmed that a genetically homologous species of P. syringae pv. syringae was responsible for bacterial blast disease of citrus cultivars. Later, PCR amplification using two primer pairs (B1 and B2) yielded 752-bp fragments to confirm that syrB gene present on both isolated P. syringae pv syringae and reference strains (Pss16) can synthesize syringomycin, a potent toxin for plant pathogenicity induction. Based on the pathogenicity tests, two strains were similar in terms of virulence activity, however, were not analogous thus, having the same level of severity effect to induce bacterial blast. Moreover, antibiotic sensitivity assay showed disproportionate result for both strains which resemble susceptible strains determination. From the in vitro investigation of six plant extracts and three Bacillus isolates against ps17a and ps18a, the ethanolic extract of Allium sativum, Moringa oleifera, and Azadirachta indica showed the highest antibacterial activity against two isolated bacterial strains up to inhibition zone 23.4, 24.4 and 18.2 mm in diameter, respectively while Bacillus isolates proved to be better natural antimicrobial agents against the disease. Finally, based on in vivo experiment, the most effective plant extracts and antagonistic isolates BS5 exhibited significant necrosis growth reduction after 10 weeks of inoculation by ps17a up to 67.48 and 52.88% as compared to 65.42 and 55.3% by ps18a, respectively. Thus, six selected ethanolic plant extracts and three isolates of Bacillus spp. have a broad-spectrum antimicrobial activity which can be used as effective biocontrol agent.
References
- Abalaka ME, Daniyan SY, Oyeleke SB, Adeyemo SO (2012) The antibacterial evaluation of Moringa oleifera leaf extracts on selected bacterial pathogens. J Microbiol Res 2(2):1–4
- Abbasi V, Rahimian H, Tajick-Ghanbari MA (2013) Genetic variability of Iranian strains of Pseudomonas syringae pv. syringae causing bacterial canker disease of stone fruits. Eur J Plant Pathol 135(2):225–235
- Abdellatif E, Kałużna M, Janse JD, Sobiczewski P, Helali F, Lamichhane JR, Rhouma A (2017) Phenotypic and genetic characterization of Pseudomonas syringae strains associated with the recent citrus bacterial blast and bacterial black pit epidemics in Tunisia. Plant Pathol 66(7):1081–1093
- Al Khateeb W, Bahar E, Lahham J, Schroeder D, Hussein E (2013) Regeneration and assessment of genetic fidelity of the endangered tree Moringa peregrina (Forsk.) Fiori using inter simple sequence repeat (ISSR). Physiol Mol Biol Plants 19(1):157–164
- Al_husnan LA, Alkahtani MDF (2016) Impact of Moringa aqueous extract on pathogenic bacteria and fungi in vitro. Ann Agric Sci 61(2):247–250
- Bakht J, Khan S, Shafi M (2014) In vitro antimicrobial activity of Allium cepa (dry bulbs) against Gram positive and Gram-negative bacteria and fungi. Pak J Pharm Sci 27(1):139–145
- Bakker P, van Peer R, Schippers B (1990) Specificity of siderophores and siderophore receptors and biocontrol by Pseudomonas spp. Biol Control Soil Borne Plant Pathogens 14:131–142
- Balestra GM, Rossetti A, Quattrucci A (2008) Biological control of kiwifruit and tomato bacterial pathogens. Poster at: cultivating the future based on science: 2nd conference of the international society of organic agriculture research ISOFAR, Modena, Italy
- Bauer AW, Kirby WMM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45(4_ts):493–496
- Beiki F, Busquets A, Gomila M, Rahimian H, Lalucat J, García-Valdés E (2016) New Pseudomonas spp. are pathogenic to citrus. PLoS One 11(2):e0148796
- Berge O, Monteil CL, Bartoli C, Chandeysson C, Guilbaud C, Sands DC, Morris CE (2014) A user’s guide to a data base of the diversity of Pseudomonas syringae and its application to classifying strains in this phylogenetic complex. PLoS One 9(9):e105547
- Besson F, Michel G (1990) Mycosubtilins B and C: minor antibiotics from mycosubtilin-producer Bacillus subtilis. Microbios 62(251):93–99
- Bradbury JF (1986) Guide to plant pathogenic bacteria. CAB International, Wallingford
- Bryan MK (1928) Lilac blight in the United States. J Agric Res 36:225–235
- Bultreys A, Gheysen I (1999) Biological and molecular detection of toxic lipodepsipeptide-producing Pseudomonas syringae strains and PCR identification in plants. Appl Environ Microbiol 65(5):1904–1909
- Bultreys A, Kaluzna M (2010) Bacterial cankers caused by Pseudomonas syringae on stone fruit species with special emphasis on the pathovars syringae and morsprunorum race 1 and race 2. J Plant Pathol 92:S21–S33
- Cappuccino JG, Sherman N (2005) Microbiology: a laboratory manual (7th edn). Pearson/Benjamin Cummings, San Francisco
- Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim S-D, Roberts DP (2008) Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl Microbiol Biotechnol 80(1):115–123
- Cirvilleri G, Bonaccorsi A, Scuderi G, Scortichini M (2005) Potential biological control activity and genetic diversity of Pseudomonas syringae pv. syringae strains. J Phytopathol 153(11–12):654–666
- Defago G, Haas D (1990) Pseudomonads as antagonists of soilborne plant pathogens: mode of actions and genetic analysis. Elsevier, Amsterdam
- fatima Douiri L, Boughdad A, Assobhei O, Moumni M (2013) Chemical composition and biological activity of Allium sativum essential oils against Callosobruchus maculatus. Toxicol Food Technol 3(1):30–36
- Fawcett HS (1936) Citrus diseases and their control, 2nd edn. Elsevier, Amsterdam
- Garrity GM (2005) Systematic bacteriology. The proteobacteria, part C: the alpha-, beta-, delta-, and epsilonproteobacteria, Bergey’s manual trust, Department of Microbiology and Molecular Genetics, vol 2. Springer, New York
- Gasic K, Prokic A, Ivanovic M, Kuzmanovic N (2015) Differentiation of Pseudomonas syringae pathovars originating from stone fruits. Pest Fitomed 27:219
- Gironde S, Manceau C (2012) Housekeeping gene sequencing and multilocus variable-number tandem-repeat analysis to identify subpopulations within Pseudomonas syringae pv. maculicola and Pseudomonas syringae pv. tomato that correlate with host specificity. Appl Environ Microbiol 78(9):3266–3279
- Gnat S, Nowakiewicz A, Ziółkowska G, Trościańczyk A, Majer-Dziedzic B, Zięba P (2017) Evaluation of growth conditions and DNA extraction techniques used in the molecular analysis of dermatophytes. J Appl Microbiol 122(5):1368–1379
- Grifoni A, Bazzicalupo M, Di Serio C, Fancelli S, Fani R (1995) Identification of Azospirillum strains by restriction fragment length polymorphism of the 16S rDNA and of the histidine operon. FEMS Microbiol Lett 127(1–2):85–91
- Guo JJ, Kuo CM, Hong JW, Chou RL, Lee YH, Chen TI (2015) The effects of garlic-supplemented diets on antibacterial activities against Photobacterium damselae subsp. piscicida and Streptococcus iniae and on growth in Cobia, Rachycentron canadum. Aquaculture 435:111–115
- Gupta D, Dubey J, Kumar M (2016) Phytochemical analysis and antimicrobial activity of some medicinal plants against selected common human pathogenic microorganisms. Asian Pac J Trop Dis 6(1):15–20
- Halebian S, Harris B, Finegold SM, Rolfe RD (1981) Rapid method that aids in distinguishing Gram-positive from Gram-negative anaerobic bacteria. J Clin Microbiol 13(3):444–448
- Hilario E, Buckley TR, Young JM (2004) Improved resolution on the phylogenetic relationships among Pseudomonas by the combined analysis of atpD, carA, recA and 16S rDNA. Antonie Van Leeuwenhoek 86(1):51–64
- Hwang MSH, Morgan RL, Sarkar SF, Wang PW, Guttman DS (2005) Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl Environ Microbiol 71(9):5182–5191
- Islam MS, Sultana R, Jahan MS, Islam F, Khalekuzzaman M, Sikdar B (2017a) Isolation and biochemical characterization of Pseudomonas syringae causing citrus blast disease and its sensitivity test against some antibiotics. Int J Sci Eng Res 8(10):2229–5518
- Islam MS, Sultana R, Khalekuzzaman M, Sikdar B, Acharjee UK, Hasan MF, Islam MA (2017b) Isolation and characterization of bacterial spot disease of citrus through biochemical approaches and its control measures. J Pharm Phytochem 6(5):2418–2422
- Ivanović Ž, Perović T, Popović T, Blagojević J, Trkulja N, Hrnčić S (2017) Characterization of Pseudomonas syringae pv. syringae, causal agent of citrus blast of mandarin in Montenegro. Plant Pathol J 33(1):21
- Jabeen R, Shahid M, Jamil A, Ashraf M (2008) Microscopic evaluation of the antimicrobial activity of seed extracts of Moringa oleifera. Pak J Bot 40(4):1349–1358
- Kałużna M, Puławska J, Sobiczewski P (2010) The use of PCR melting profile for typing of Pseudomonas syringae isolates from stone fruit trees. Eur J Plant Pathol 126(4):437–443
- Kaur T, Jasrotia S, Ohri P, Manhas RK (2016) Evaluation of in vitro and in vivo nematicidal potential of a multifunctional streptomycete, Streptomyces hydrogenans strain DH16 against Meloidogyne incognita. Microbiol Res 192:247–252
- Lamichhane JR (2015) Bacterial diseases of crops: elucidation of the factors that lead to differences between field and experimental infections. Advances in agronomy, vol 134. Elsevier, Amsterdam, pp 227–246
- Lamichhane JR, Varvaro L, Parisi L, Audergon J-M, Morris CE (2014) Disease and frost damage of woody plants caused by Pseudomonas syringae: seeing the forest for the trees. Advances in agronomy, vol 126. Elsevier, Amsterdam, pp 235–295
- Lelliott RA, Billing E, Hayward AC (1966) A determinative scheme for the fluorescent plant pathogenic pseudomonads. J Appl Bacteriol 29(3):470–489
- Logaranjan K, Devi S, Pandian K (2012) Biogenic synthesis of silver nanoparticles using Fruit extract of Ficus carica and study its antimicrobial activity. Nano Biomed Eng 4(4):177
- Louws FJ, Fulbright DW, Stephens CT, De Bruijn FJ (1994) Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl Environ Microbiol 60(7):2286–2295
- Lu S, Tian Q, Zhao W, Hu B (2017) Evaluation of the potential of five housekeeping genes for identification of quarantine Pseudomonas syringae. J Phytopathol 165(2):73–81
- MacConkey A (1905) Lactose-fermenting bacteria in faeces. Epidemiol Infect 5(3):333–379
- Mahboubi A, Asgarpanah J, Sadaghiyani PN, Faizi M (2015) Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. var. pleniflora flowers (Golnar) against bacterial strains causing foodborne diseases. BMC Complement Alternat Med 15(1):366
- Mahfuzul Hoque MD, Bari ML, Inatsu Y, Juneja VK, Kawamoto S (2007) Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathogens Dis 4(4):481–488
- Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P et al (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13(6):614–629
- Mari M, Neri F, Bertolini P (2007) Novel approaches to prevent and control postharvest diseases of fruits. Stewart Postharvest Rev 3(6):1–7
- Mirik M, Baloglu S, Aysan Y, Cetinkaya-Yildiz R, Kusek M, Sahin F (2005) First outbreak and occurrence of citrus blast disease, caused by Pseudomonas syringae pv. syringae, on orange and mandarin trees in Turkey. Plant Pathol 54(2):238
- Miron T, Shin I, Feigenblat G, Weiner L, Mirelman D, Wilchek M, Rabinkov A (2002) A spectrophotometric assay for allicin, alliin, and alliinase (alliin lyase) with a chromogenic thiol: reaction of 4-mercaptopyridine with thiosulfinates. Anal Biochem 307(1):76–83
- Monteiro L, de Mariano RLR, Souto-Maior AM (2005) Antagonism of Bacillus spp. against Xanthomonas campestris pv. campestris. Braz Arch Biol Technol 48(1):23–29
- Morris CE, Sands DC, Vanneste JL, Montarry J, Oakley B, Guilbaud C, Glaux C (2010) Inferring the evolutionary history of the plant pathogen Pseudomonas syringae from its biogeography in headwaters of rivers in North America, Europe, and New Zealand. MBio 1(3):e00107–e00110
- Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 25(2):361–366
- Mougou I, Boughalleb-M’hamdi N (2018) Biocontrol of Pseudomonas syringae pv. syringae affecting citrus orchards in Tunisia by using indigenous Bacillus spp. and garlic extract. Egypt J Biol Pest Control 28(1):1–11
- Naqvi SF, Inam-ul-Haq M, Tahir MI, Mughal SM (2012) Screening of sesame germplasm for resistance against the bacterial blight caused by Xanthomonas campestris pv. sesami. Pak J Agric Sci 49(2):131–134
- Parke JL, Gurian-Sherman D (2001) Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 39(1):225–258
- Peypoux F, Bonmatin JM, Wallach J (1999) Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 51(5):553–563
- Philip K, Malek SNA, Sani W, Shin SK, Kumar S, Lai HS et al (2009) Antimicrobial activity of some medicinal plants from Malaysia. Am J Appl Sci 6(8):1613
- Qader MK, Khalid NS, Abdullah AM (2013) Antibacterial activity of some plant extracts against clinical pathogens. Int J Microbiol Immunol Res 1(5):53–56
- Rajni J, Kailash A (2011) Bio-efficacy of plant extracts against Pseudomonas syringae pv. syringae causing leaf spot of cluster bean. Ann Plant Prot Sci 19(1):106–112
- Rajwar A, Sahgal M (2016) Phylogenetic relationships of fluorescent pseudomonads deduced from the sequence analysis of 16S rRNA, Pseudomonas-specific and rpoD genes. 3 Biotech 6(1):80
- Schaad NW, Azad H, Peet RC, Panopoulos NJ (1989) Identification of Pseudomonas syringae pv. phaseolicola by a DNA hybridization probe. Probe 79903:907
- Schmidt CS, Lorenz D, Wolf GA, Jäger J (2001) Biological control of the grapevine dieback fungus Eutypa lata II: influence of formulation additives and transposon mutagenesis on the antagonistic activity of Bacillus subtilis and Erwinia herbicola. J Phytopathol 149(7–8):437–445
- Scortichini M, Marchesi U, Dettori MT, Rossi MP (2003) Genetic diversity, presence of the syrB gene, host preference and virulence of Pseudomonas syringae pv. syringae strains from woody and herbaceous host plants. Plant Pathol 52(3):277–286
- Sessitsch A, Reiter B, Berg G (2004) Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Can J Microbiol 50(4):239–249
- Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31(3):446–459
- Sorensen KN, Kim K-H, Takemoto JY (1998) PCR detection of cyclic lipodepsinonapeptide-producing Pseudomonas syringae pv. syringae and similarity of strains. Appl Environ Microbiol 64(1):226–230
- Srinivasan D, Nathan S, Suresh T, Perumalsamy PL (2001) Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol 74(3):217–220
- Srivastava R, Ghosh S, Mandal DB, Azhahianambi P, Singhal PS, Pandey NN, Swarup D (2008) Efficacy of Azadirachta indica extracts against Boophilus microplus. Parasitol Res 104(1):149–153
- Suslow TV, Schroth MN, Isaka M (1982) Application of a rapid method for Gram differentiation of plant pathogenic and saprophytic bacteria without staining. Phytopathology (USA) 72:917
- Tabbene O, Slimene IB, Bouabdallah F, Mangoni M-L, Urdaci M-C, Limam F (2009) Production of anti-methicillin-resistant Staphylococcus activity from Bacillus subtilis sp. strain B38 newly isolated from soil. Appl Biochem Biotechnol 157(3):407–419
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729
- Thornberry HH (1950) A paper-disk plate method for the quantitative evaluation of fungicides and bactericides. Phytopathology 40(5):419
- Toure Y, Ongena M, Jacques P, Guiro A, Thonart P (2004) Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J Appl Microbiol 96(5):1151–1160
- Tripathi P, Dubey NK, Shukla AK (2008) Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J Microbiol Biotechnol 24(1):39–46
- Vanneste JL (2000) Fire blight: the disease and its causative agent, Erwinia amylovora. CABI, Willingford
- Vasebi Y, Khakvar R, Faghihi MM, Vinatzer BA (2019) Genomic and pathogenic properties of Pseudomonas syringae pv. syringae strains isolated from apricot in East Azerbaijan province, Iran. Biocatal Agric Biotechnol 19:101167
- Verma V, Singh R, Tiwari RK, Srivastava N, Verma A (2012) Antibacterial activity of extracts of Citrus, Allium and Punica against food borne spoilage. Asian J Plant Sci Res 2(4):503–509
- Völksch B, Weingart H (1998) Toxin production by pathovars of Pseudomonas syringae and their antagonistic activities against epiphytic microorganisms. J Basic Microbiol Int J Biochem Physiol Genet Morphol Ecol Microorgan 38(2):135–145
- Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA et al (2011) Bergey’s manual of systematic bacteriology: the Firmicutes, vol 3. Springer Science & Business Media, New York
- Wikaningtyas P, Sukandar EY (2016) The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens. Asian Pac J Trop Biomed 6(1):16–19
- Yamamoto S, Kasai H, Arnold DL, Jackson RW, Vivian A, Harayama S (2000) Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. The GenBank accession numbers for the sequences determined in this work are: gyrB, D37926, D37297, D86005–D86019 and AB039381–AB03. Microbiology 146(10):2385–2394
- Young JM (1991) Pathogenicity and identification of the lilac pathogen, Pseudomonas syringae pv. syringae van Hall 1902. Ann Appl Biol 118(2):283–298
- Zam SI, Syamsuardi AA, Jannah M, Aldi Y, Djamaan A (2016) Isolation, characterization of endophytic bacteria from Citrus aurantifolia swingle leaves and testing of antifungal activity towards Fusarium oxysporum. Der Pharm Lett 8(11):83–89
Author Information
Department of Plant Pathology, College of Plant Science and Technology and the Key Lab of Crop Disease Monitoring & Safety Control in Hubei Province, Huazhong Agricultural University, Wuhan, China