Characterization of nitrilase-producing thermophilic bacteria and exploring their potential for mandelic acid synthesis
Research Articles | Published: 14 March, 2023
First Page: 257
Last Page: 265
Views: 3621
Keywords: Thermophile, Nitrilase, n Geobacillusn , Mandelic acid, Enantioselectivity
Abstract
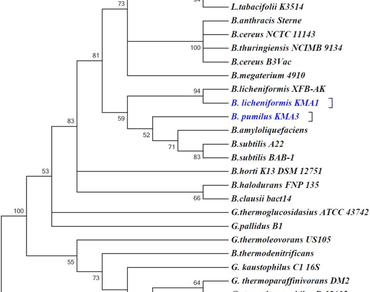
The present study was undertaken to screen thermophilic nitrilase-producing bacteria from the hot water springs of Manikaran and Kheerganga, located in the Kullu District of Himachal Pradesh, India, to harness mandelic acid from mandelonitrile. Five nitrile degrading isolates from these thermophilic sites exhibiting appreciable mandelonitrile degrading ability were subjected to biochemical characterization, and all bacteria were gram-positive rods. Molecular characterization was done by 16s rDNA sequencing, and the phylogenetic tree depicted their relatedness. There were three bacteria: Bacillus licheniformis KMA 1, Lysinibacillus macroides KMA 2, and Bacillus pumilus KMA 3 from Kheerganga. On the other hand, two promising bacteria were obtained from the Manikaran hotspring, designated as Geobacillus stearothermophilus MAC I and Geobacillus icigianus MAC VI, displayed high thermotolerance and suitably grew at 60 °C. Geobacillus strains showed a similar enzymatic profile when tested against mandelonitrile as substrate. Therefore, only one strain, G. icigianus MAC VI, was selected for optimization and scale-up studies. Even at elevated temperatures of 100 °C, the enzyme retained its enzymatic prowess and could effectively degrade mandelonitrile; however, a dip in enzyme activity was also concurrently recorded. The standardization of process parameters further resulted in a 7.4-fold increase in the nitrilase activity of the selected strain. Furthermore, a delayed inducer feeding strategy was also incorporated to cause hyper-induction of nitrilase enzyme effectively. Scale-up production of mandelic acid was carried out in a batch reaction that yielded 8.4 g of R-mandelic acid with 74%ee.
References
Agarwal A, Nigam VK, Vidyarthi A (2012) Nitrilases- an attractive nitrile degrading biocatalyst. Int J Pharma Bio Sci 3(4):232–246
Akmar HN, Asma I, Venugopal B, Atha LY, Sasidharan S (2011) Identification of appropriate sample and culture method for isolation of new thermophilic bacteria from hot spring. Afr J Microbiol Res 5:217–221
Banerjee A, Kaul P, Sharma R, Banerjee UC (2003) A high-throughput amenable colorimetric assay for enantioselective screening of nitrilase-producing microorganisms. J BioMol Screen 8:559–565
Bhatia K, Mal G, Bhar R, Jyoti, Attri C, Seth A (2018) Purification and characterization of thermostable superoxide dismutase from Anoxybacillus gonensis KA 55 MTCC 12684. Int J Biol Macromol 117:1133–1139. https://doi.org/10.1016/j.ijbiomac.2018.06.031
Buchholz K, Kasche V, Bornscheuer UT (2012) Biocatalysts and enzyme technology, 2nd edn. Wiley-VCH
Chauhan S, Seth CA, Seth A (2015) Bioprospecting thermophilic microorganisms from hot springs of western himalayas for xylanase production and its statistical optimization by using response surface methodology. J Pure Appl Microbiol 9(2):1417–1428
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159. https://doi.org/10.1136/jcp.13.2.156
Gong JS, Lu ZM, Li H, Shi JS, Zhou ZM, Xu ZH (2012) Nitrilases in nitrile biocatalysis: recent progress and forthcoming research. Microb Cell Fact 11:142
Jiang W, Tao R, Yang Y, Xu Y, Kang H, Zhou X, Zhou Z (2017) Production of (R)-(–)-mandelic acid with nitrilase immobilized on D155 resin modified by L-lysine. Biochem Eng J. https://doi.org/10.1016/j.bej.2017.07.010
Jyoti, Bhatia K, Chauhan K, Attri C, Seth A (2017) Improving stability and reusability of Rhodococcus pyridinivorans NIT-36 nitrilase by whole cell immobilization using chitosan. Int J Biol Macromol 103:8–15. https://doi.org/10.1016/j.ijbiomac.2017.05.012
Kimura M, Kuboki A, Sugai T (2002) Chemo-enzymatic synthesis of enantiomerically pure (R)-2-naphthylmethoxyacetic acid. Tetrahedron Asymmetry 13:1059–1068. https://doi.org/10.1016/S0957-4166(02)00242-2
Kohilu U, Nigam P, Singh D, Choudhary DK (2008) Thermostable alkalophilic and cellulase free xylanase production by Thermoactinomyces thalophilus subgroup C. Enzym Microb Technol 28:606–610
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Kumari A, Singh P, Seth AC, Seth A (2016) Batch and fed-batch production of acetohydroxamic acid using amidase of hyperinduced cells of Rhodococcus pyridinivorans NIT-36. Res J Chem Environ 20(7):35–47
Liu Z, Dong L, Cheng F, Xue Y, Wang S, Ding J, Zheng Y, Shen Y (2011) Gene cloning, expression and characterization of a nitrilase from Alcaligenes faecalis ZJUTB10. J Agric Food Chem 59:11560–11570. https://doi.org/10.1021/jf202746a
Liu ZQ, Zhang XH, Xue YP, Xu M, Zheng YG (2014) Improvement of Alcaligenes faecalis nitrilase by gene site saturation mutagenesis and its application in stereospecific biosynthesis of (R)-(-)-mandelic acid. J Agric Food Chem 62(20):4685–4694. https://doi.org/10.1021/jf405683f
Mori K, Akao H (1980) Synthesis of optically active alkynyl alcohols and alpha-hydroxy esters by microbial asymmetric hydrolysis of the corresponding acetates. Tetrahedron 36(1):91–96. https://doi.org/10.1016/0040-4020(80)85030-7
Pandey AC, Durve AA, Pathak MS, Sharon M (2011) White biotech approach to synthesize mandelic acid using microbes and plants as a source of nitrilase. Asian J Exp Biol Sci 29(1):191–200
Pratush A, Seth A, Bhalla TC (2012) Cloning, sequencing and expression of nitrile hydratase gene of mutant 4D strain of Rhodococcus rhodochrous PA 34 in E. coli. Applied Biochemistry and Biotechnology 168:465–486. https://doi.org/10.1007/s12010-012-9790-9
Raj J, Singh N, Prasad S, Seth A, Bhalla TC (2007) Bioconversion of benzonitrile to benzoic acid using free and agar entrapped cells of Nocardia globerulaNHB-2. Acta Microbiol Immunol Hung 54(1):79–88. https://doi.org/10.1556/amicr.54.2007.1.8
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Santoshkumar M, Nayak AS, Anjaneya O, Timmanagouda B, Karegoudar TB (2010) A plate method for screening of bacteria capable of degrading aliphatic nitriles. J Ind Microbiol Biotechnol 37:111–115. https://doi.org/10.1007/s10295-009-0663-3
Singh P, Kumari A, Attri C, Seth A (2019) Efficient lactamide synthesis by fed-batch method using nitrile hydratase of Rhodococcus pyridinivoransNIT-36. J Microbiol Biotechnol Food Sci 9(3):567–572. https://doi.org/10.15414/jmbfs.2019/20.9.3.5-572
Strauss UT, Faber K (1999) Deracemization of (±)-mandelic acid using a lipase-mandelate racemase two-enzyme system. Tetrahedron: Asymmetry 10(21):4079–4081. https://doi.org/10.1016/S0957-4166(99)00436-X
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA), 101:11030–11035. https://doi.org/10.1073/pnas.0404206101
Wang H, Sun H, Gao W, Wei D (2013) Efficient production of (R)-o-chloromandelic acid by recombinant Escherichia coli cells harboring nitrilase from Burkholderia cenocepacia J2315.Org. Proc Res Dev 18:767–773
Williams G, Stickler D (2007) Some observations on the diffusion of antimicrobial agents through the retention balloons of foley catheters. J Urol 178(2):697–701. https://doi.org/10.1016/j.juro.2007.03.091
Xue YP, Xu M, Chen HS, Liu ZQ, Wang YJ, Zheng YG (2013) A novel integrated bioprocess for efficient production of (R)-(-)-mandelic acid with immobilized Alcaligenes faecalis ZJUTB10. Org Proc Res Dev 17:213–220
Zeigler DR (2014) The Geobacillus paradox: why is a thermophilic bacterial genus so prevalent on a mesophilic planet? https://doi.org/10.1099/mic.0.071696-0. Microbiology (UK)
Zhang ZJ, Xu JH, He YC, Ouyang LM, Liu YY (2010) Cloning and biochemical properties of a highly thermostable and enantioselective nitrilase from Alcaligenes sp. ECU0401 and its potential for (R)-(–)-mandelic acid production. Bioprocess Biosyst Eng 34:315–322. https://doi.org/10.1007/s00449-010-0473-z
Author Information
School of Biotechnology, Sher-e-Kashmir University of Agricultural Sciences & Technology of Jammu, Chatha, Jammu, India