Cypermethrin toxicity to rice field cyanobacterium Calothrix sp.
Research Articles | Published: 18 May, 2020
First Page: 401
Last Page: 408
Views: 3994
Keywords: Biomass, Nitrogen fixation, Pesticide, Cypermethrin, Calothrix sp.
Abstract
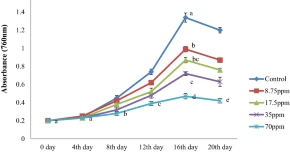
Cyanobacteria are one of the potent group of microbes in wet land soils, especially in rice fields. Most of them have the capacity to fix atmospheric nitrogen and thus play a crucial role in nitrogen budget of soil where they grow. With the advent of green revolution these microbes have been gradually exposed to pesticides in large scale that poses a great threat to them. The present study was undertaken to investigate the influence of cypermethrin, a pyrethroid insecticide, on the growth and physiological activities of a rice field nitrogen fixing cyanobacterium Calothrix sp. (strain GUEco 1003) under controlled laboratory conditions. The test cyanobacterium showed varying degree of sensitivity against cypermethtin. To evaluate the toxicity, the organism was exposed to varying concentrations of the insecticide (8.75–70 ppm) based on LC50 for a period of 20 days. Results revealed that cypermethrin negatively affected its growth (65%), biomass (67%), chlorophyll-a (68%), protein (53%) and nitrogen content (65%) in a time-dose dependent manner. However the organism showed increase carbohydrate content with the increasing concentration of the insecticide over the control. Reduction in growth, biomass, chlorophyll-a, protein and nitrogen content with the increasing concentration of cypermethrin was an indication of its toxicity to the Calothrix sp., which is one of the natural biofertilizer in any agricultural field.
References
- Adhikary SP (1983) Growth measurements by monitoring light scattering of a filamentous blue green alga which does not give uniform and stable suspension in culture vessels. Zeitschrifts fur Allg Mikrobiologie 23:475–483
- Adhikary SP (1989) Effect of pesticides on growth, photosynthetic oxygen evolution and nitrogen fixation of Westiellopsis prolifica. J Gen App Microbiol 35:319–325
- Averamova S, Rossler M (1975) Effect of various temperatures on some physiological–biochemical induces during the light phase of the life cycle of Scenedesmus sp. Appl Microbiol 5:115–120
- Bhosle NP, Nasreen S (2013) Remediation of cypermethrin- 25EC by microorganisms. Eur J Exp Biol 3(1):144–152
- Chen T, Huang X, Guo et al (2013) Butachlor induced some physiological and biochemical changes in a rice field biofertilizer cyanobacterium. Pestic Biochem Phys 105:24–30
- Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi, p 686
- Dowidar SMA, Osman MEH, Naggar AHEI, Khalefa AE (2010) Effect of butachlor and thiobencarb herbicides on protein content and profile and some enzyme activities of Nostoc muscorum. J Genet Eng Biotechnol 8:89–95
- Gafur MA, Parvin S (2008) Distribution of Blue green algae in soils of Chittagong University Campus and their nitrogen fixing capacity. Bangladesh J Bot 37(1):49–53
- Galhano V, Peixoto F, Gomes-Laranjo J, Fernandez-Valiente E (2009) Differential effect of bentazon and molinate on Anabaena cylindrica, an autochthonous cyanobacterium of Portuguese rice field agro ecosystem. Water Air Soil Pollut 197(1–4):2112–222
- Guillard RL (1973) Division rates. In: Stein JR (ed) Handbook of physiological methods, culture methods and growth measurement. Cambridge University Press, New York, pp 290–331
- Gupta K, Baruah PP (2015) Effect of lambdacyhalothrin on Calothrix sp. (GUEco 1001), an authchthonous cyanobacterium of rice fields of Brahmaputra floodplain. Environ Sci Pollut Res 22:18554–18560
- Gupta K. Baruah PP (2017) Isolation, identification and characterization of rice field Calothrix spp. of Assam. J Algal Biomass Utln 8(4):77–81
- Habib K, Manikar N, Ansari S, Fatma T (2013) Carbaryl stress induced cellular changes in Calothrix brevissima. Environ Sci Pollut Res 20:862–871
- Hashtroudi MS, Ghassempour A, Riahi H, Shariatmadari Z, Khanjir M (2013) Endogenous auxins in plant growth-promoting Cyanobacteria: Anabaena vaginicola and Nostoc calcicola. J Appl Phycol 25:379–386
- Huang TC, Chow TJ (1992) Characterzation of the Calothrix isolates from rice fields. Bot Bull Acad Sin 33:23–31
- Kiran G, Sharma SG, Singh SP (2006) Effects of monocrotophos and butachlor on N-fixing cyanobacteria and associated biochemical activities. Ann Plant Protect Sci 14(1):210–214
- Komarek J, Anagnostidis K (1989) Modern approach to the classification system of Cyanophytes 4- Nostocales. Algol Stud 56:247–345
- Kumar S, Habib K, Fatma T (2008) Endosulfan induced biochemical changes in nitrogen fixing cyanobacteria. Sci Total Environ 403(1–3):130 –130 38
- Kumar JIN, Kumar RN, Bora A, Kaur AM (2011) An evaluation of pesticides stress induces proteins in three cyanobacterial species: Anabaena fertilissima, Aulosira fertilissima, Westiellopsis prolifica using SDS–PAGE. Adv Environ Biol 5(4):739–745
- Kumar J, Singh R, Parihar P, Singh VP, Prasad SM (2016) UV-B induces biomass production and nonenzymatic antioxidant compounds in three cyanobacteria. J Appl Phycol 28:131–140
- Lal S, Saxena DM (1980) Cytological and biochemical effect of pesticides on microorganisms. Residue Rev 73:49–86
- Lowry OH, Rosenbrough NJ, Farr AI, Randal RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–276
- Mackinney G (1941) Absorbtion of light by chlorophyll solution. J Biol Chem 140:315–322
- Megharaj M, Venkateswarlu K, Rao AS (1987) Influence of cypermethrin and fenvalerate on a green alga and three cyanobacteria isolated from soil. Ecotoxicol Environ Saf 14(2):142–146
- Mohapatra PK, Patra S, Samantaray PK, Mohanty RC (2003) Effect of the pyrethroid insecticide cypermethrin on photosynthetic pigments of the cyanobacterium Anabaena doliolum Bhar. Pollut J Environ Stud 12(2):207–212
- Okmen G, Ugur A (2011) Influence of bispyribac sodium on nitrogenase activity and growth of cyanobacteria isolated from paddy fields. Afr J Microbiol Res 5(18):2760–2764
- Padhy RN (1985) Cyanobacteria and pesticides. Residue Rev 95:1–44
- Pimentel D (1995) Amounts of pesticides reaching target pests: environmental impacts and ethics. J Agric Environ Ethic 8:17–29
- Prasad SM, Sheeba SVP, Srivastava PK (2011) Differential physiological and biochemical responses of two cyanobacteria Nostoc muscorum and Phormidium foveolarum against oxyfluorfen and UV-B radiation. Ecotoxicol Environ Saf 74:1981–1993
- Rajendran UM, Kathirvel E, Narayanaswamy A (2007) Effects of a fungicide, an insecticide and a biopesticide on Tolypothrix sctynemoides. Pestic Biocheme Physiol 87:164–171
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stainer RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
- Roger PA, Kulasooriya SA (1980) Blue green algae and rice. IRRI Los Banos, Philippines, p 112
- Sahu D, Bastia AK, Rath B (2015) Toxicity of organophosphorus pesticides on rice field cyanobacteria. Int J Geol 3(6):6–10
- Shen JY, Jiang J, Zheng P (2009) Effect of light and monosulfuron on growth and photosynthetic pigments of Anabaena flos aquae Breb. J Water Resour Prot 1:408–413
- Shinde GS, Pingle D, Gunale VR (2010) Interaction of Calothrix javanica de Wilde with furadan, a carbomate pesticide. Asian J Exp Biol Sci Spl 1(1):129–131
- Singh DP, Khattar JIS, Gupta M, Kaur G (2014) Evaluation of toxicological impact of cartap hydrochloride on some physiological activities of a non-heterocystous cyanobacterium Leptolyngbya foveolarum. Pestic Biochem Phys 110:63–70
- Singh DP, Khattar JIS, Alka GK, Singh Y (2016) Toxicological effect of pretilachlor on some physiological processes of cyanobacterium Synechocystis sp. strain PUPCCC 64. J Appl Biol Biotech 4(01):012–019
- Spiro RG (1966) Analysis of sugars found in glycoproteins. Methods enzymol 8:3–26
- Srinivasulu M, Rangaswamy V (2013) Influence of insecticides alone and in combination with fungicides on enzyme activities in soil. Int J Environ Sci Technol 10:341–350
- Stewart WDP, Rowell P, Kerby NW, Reed RH, Machray GC (1987) A century of nitrogen fixation. Phil Trans R Soc Lond 317:245–258
- Tiwari SP, Sharma NK, Tripathi K, Rai AK (2011) Sustainability and Cyanobacteria (blue green algae): facts and challenges. J Appl Phycol 23:1059–1081
- Xia J (2005) Response of Growth, photosynthesis and photoinhibition of the edible cyanobacterium Nostoc sphaeriodes colonies to thiobencarb herbicides. Chemosphere 59:561–566
- Yadav NR, Sharma S (2013) Toxic effect of organophosphate, pyrethroids and organochlorine pesticides on Spirulina platensis growth rate. Int J Sci Res 2(6):286–287
- Yoshida S, Forno DA, Cock DH, Gomez KA (1976) Laboratory manual for physiological studies of rice, 3rd edn. The international Rice Research Institute, Los Banos, p 83
Author Information
Department of Botany, Gauhati University, Guwahati, India