De novo transcriptome sequencing of Monodopsis subterranea CCALA 830 and identification of genes involved in the biosynthesis of eicosapentanoic acid and triacylglycerol
Research Articles | Published: 16 September, 2019
First Page: 600
Last Page: 608
Views: 4236
Keywords: Monodopsis subterranea , Transcriptome, Illumina HiSeq 2000, Fatty acid biosynthesis, TAG, EPA
Abstract
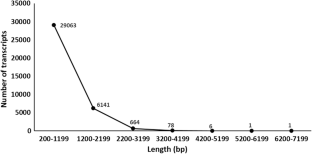
Monodopsis subterranea, a unicellular yellow-green freshwater microalga, is widely known for its ability to produce high amount of therapeutically beneficial ω-3 polyunsaturated fatty acid eicosapentanoic acid (EPA; C20:5Δ5,8,11,14,17). Currently, no genomic information is available on M. subterranea despite its nutraceutical and commercial applications. Analysis on fatty acid methyl esters from M. subterranea strain CCALA 830 demonstrated accumulation of 28% EPA. In order to obtain better understanding of EPA biosynthesis and to identify genes involved in the process of lipid metabolism and accumulation in this alga, de novo transcriptome sequencing and assembly was performed using Illumina Hiseq 2000 sequencing. A total of 35,954 transcripts were obtained through final transcriptome assembly with an average transcript length of 769.36 bp. BLAST similarity searches for assembled transcripts were performed followed by annotation using Gene ontology and Kyoto Encyclopedia of Genes and Genomes orthology identifiers. Transcripts involved in lipid biosynthesis including various fatty acid desaturases and elongases involved in PUFAs biosynthesis were identified during the study. In addition, sequences for several transcription factors and a number of simple sequence repeats were also ascertained which can be used as powerful genetic markers for further genetic analysis. This study would provide a useful resource for future research on M. subterranea genome.
References
- Abdellaoui N, Kim MJ, Choi TJ (2019) Transcriptome analysis of gene expression in Chlorella vulgaris under salt stress. World J Microbiol Biotechnol 35:141. https://doi.org/10.1007/s11274-019-2718-6
- Altschul SF, Madden TL, Schaffer AA et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
- Arao T, Sakaki T, Yamada M (1994) Biosynthesis of polyunsaturated lipids in the diatom, Phaeodactylum tricornutum. Phytochemistry 36:629–635. https://doi.org/10.1016/S0031-9422(00)89787-3
- Arora S, Mishra G (2019) Biochemical modulation of Monodopsis subterranea (Eustigmatophyceae) by auxin and cytokinin enhances eicosapentaenoic acid productivity. J Appl Phycol. https://doi.org/10.1007/s10811-019-01844-3
- Chu F, Cheng J, Zhang X et al (2019) Transcriptome and key gene expression related to carbon metabolism and fatty acid synthesis of Chlorella vulgaris under a nitrogen starvation and phosphorus repletion regime. J Appl Phycol. https://doi.org/10.1007/s10811-019-01811-y
- Cohen Z (1994) Production potential of eicosapentaenoic acid by Monodus subterraneus. J Am Oil Chem Soc 71:941–945. https://doi.org/10.1007/BF02542258
- Conesa A, Götz S, García-Gómez JM et al (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676. https://doi.org/10.1093/bioinformatics/bti610
- Corteggiani Carpinelli E, Telatin A, Vitulo N et al (2014) Chromosome scale genome assembly and transcriptome profiling of Nannochloropsis gaditana in nitrogen depletion. Mol Plant 7:323–335. https://doi.org/10.1093/mp/sst120
- Ewing B, Green P (1998) Base-calling of automated sequencer traces using phred. Genome Res 8:186–194. https://doi.org/10.1101/gr.8.3.186
- Fukuda S, Hirasawa E, Takemura T et al (2018) Accelerated triacylglycerol production without growth inhibition by overexpression of a glycerol-3-phosphate acyltransferase in the unicellular red alga Cyanidioschyzon merolae. Sci Rep. https://doi.org/10.1038/s41598-018-30809-8
- Gao C, Wang Y, Shen Y et al (2014) Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genomics. https://doi.org/10.1186/1471-2164-15-582
- Grabherr MG, Haas BJ, Yassour M et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652. https://doi.org/10.1038/nbt.1883
- Guarnieri MT, Nag A, Smolinski SL et al (2011) Examination of triacylglycerol biosynthetic pathways via de novo transcriptomic and proteomic analyses in an unsequenced microalga. PLoS One 6:25851. https://doi.org/10.1371/journal.pone.0025851
- Haas BJ, Papanicolaou A, Yassour M et al (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512. https://doi.org/10.1038/nprot.2013.084
- He M, Song H, Chen W et al (2019) Comparative transcriptome analysis of wild type and an oleaginous mutant strain of Desmodesmus sp. reveals a unique reprogramming of lipid metabolism under high light. J Appl Phycol. https://doi.org/10.1007/s10811-019-01821-w
- Hibberd DJ (1981) Notes on the taxonomy and nomenclature of the algal classes Eustigmatophyceae and Tribophyceae (synonym Xanthophyceae). Bot J Linn Soc 82:93–119. https://doi.org/10.1111/j.1095-8339.1981.tb00954.x
- Kaye Y, Grundman O, Leu S et al (2015) Metabolic engineering toward enhanced LC-PUFA biosynthesis in Nannochloropsis oceanica: overexpression of endogenous δ12 desaturase driven by stress-inducible promoter leads to enhanced deposition of polyunsaturated fatty acids in TAG. Algal Res 11:387–398. https://doi.org/10.1016/j.algal.2015.05.003
- Khozin-Goldberg I, Didi-Cohen S, Shayakhmetova I, Cohen Z (2002) Biosynthesis of eicosapentaenoic acid (EPA) in the freshwater eustigmatophyte Monodus subterraneus (Eustigmatophyceae). J Phycol 38:745–756. https://doi.org/10.1046/j.1529-8817.2002.02006.x
- Koid AE, Liu Z, Terrado R et al (2014) Comparative transcriptome analysis of four prymnesiophyte algae. PLoS One. https://doi.org/10.1371/journal.pone.0097801
- Kuntal H, Sharma V, Daniell H (2012) Microsatellite analysis in organelle genomes of Chlorophyta. Bioinformation 8:255–259. https://doi.org/10.6026/97320630008255
- Lauritano C, De Luca D, Ferrarini A et al (2017) De novo transcriptome of the cosmopolitan dinoflagellate Amphidinium carterae to identify enzymes with biotechnological potential. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-12092-1
- Li W, Godzik A (2006) Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22:1658–1659. https://doi.org/10.1093/bioinformatics/btl158
- Li H, Wang W, Wang Z et al (2016a) De novo transcriptome analysis of carotenoid and polyunsaturated fatty acid metabolism in Rhodomonas sp. J Appl Phycol 28:1649–1656. https://doi.org/10.1007/s10811-015-0703-5
- Li L, Zhang G, Wang Q (2016b) De novo transcriptomic analysis of Chlorella sorokiniana reveals differential genes expression in photosynthetic carbon fixation and lipid production. BMC Microbiol. https://doi.org/10.1186/s12866-016-0839-8
- Liang C, Cao S, Zhang X et al (2013) De novo sequencing and global transcriptome analysis of Nannochloropsis sp. (Eustigmatophyceae) following nitrogen starvation. Bioenergy Res 6:494–505. https://doi.org/10.1007/s12155-012-9269-0
- Liu B, Benning C (2013) Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotechnol 24:300–309. https://doi.org/10.1016/j.copbio.2012.08.008
- Liu CP, Lin LP (2005) Morphology and eicosapentaenoic acid production by Monodus subterraneus UTEX 151. Micron 36:545–550. https://doi.org/10.1016/j.micron.2005.05.001
- Lv H, Qu G, Qi X et al (2013) Transcriptome analysis of Chlamydomonas reinhardtii during the process of lipid accumulation. Genomics 101:229–237. https://doi.org/10.1016/j.ygeno.2013.01.004
- Mansfeldt CB, Richter LV, Ahner BA et al (2016) Use of de novo transcriptome libraries to characterize a novel oleaginous marine Chlorella species during the accumulation of triacylglycerols. PLoS One. https://doi.org/10.1371/journal.pone.0147527
- Meesapyodsuk D, Qiu X (2012) The front-end desaturase: structure, function, evolution and biotechnological use. Lipids 47:227–237. https://doi.org/10.1007/s11745-011-3617-2
- Merchant SS, Kropat J, Liu B et al (2012) TAG, You’re it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Curr Opin Biotechnol 23:352–363. https://doi.org/10.1016/J.COPBIO.2011.12.001
- Patel RK, Jain M (2012) NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLoS One 7:30619. https://doi.org/10.1371/journal.pone.0030619
- Peng KT, Zheng CN, Xue J et al (2014) Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. J Agric Food Chem 62:8773–8776. https://doi.org/10.1021/jf5031086
- Riaño-Pachón D, Ruzicic S, Dreyer I, Mueller-Roeber B (2007) PlnTFDB: an integrative plant transcription factor database. BMC Bioinform 8:42. https://doi.org/10.1186/1471-2105-8-42
- Rismani-yazdi H, Haznedaroglu BZ, Bibby K, Peccia J (2011) Transcriptome sequencing and annotation of the microalgae Dunaliella tertiolecta: pathway description and gene discovery for production of next-generation biofuels. BMC Genomics 12:148. https://doi.org/10.1186/1471-2164-12-148
- Rismani-Yazdi H, Haznedaroglu BZ, Hsin C, Peccia J (2012) Transcriptomic analysis of the oleaginous microalga Neochloris oleoabundans reveals metabolic insights into triacylglyceride accumulation. Biotechnol Biofuels 5:74. https://doi.org/10.1186/1754-6834-5-74
- Santos LM, Leedale GF (1995) Some notes on the ultrastructure of small azoosporic members of the algal class Eustigmatophyceae. Nov Hedwigia 60:219–225
- Sato A, Matsumura R, Hoshino N et al (2014) Responsibility of regulatory gene expression and repressed protein synthesis for triacylglycerol accumulation on sulfur-starvation in Chlamydomonas reinhardtii. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00444
- Schroeder A, Mueller O, Stocker S et al (2006) The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. https://doi.org/10.1186/1471-2199-7-3
- Tautz D, Renz M (1984) Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res 12:4127–4138. https://doi.org/10.1093/nar/12.10.4127
- Thiel T, Michalek W, Varshney RK, Graner A (2003) Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor Appl Genet 106:411–422. https://doi.org/10.1007/s00122-002-1031-0
- Thiriet-Rupert S, Carrier G, Chénais B et al (2016) Transcription factors in microalgae: genome-wide prediction and comparative analysis. BMC Genomics. https://doi.org/10.1186/s12864-016-2610-9
- Torre S, Tattini M, Brunetti C et al (2016) De Novo assembly and comparative transcriptome analyses of red and green morphs of sweet basil grown in full sunlight. PLoS One 11:1–19. https://doi.org/10.1371/journal.pone.0160370
- Vieler A, Wu G, Tsai C-H et al (2012) Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet 8:1003064. https://doi.org/10.1371/journal.pgen.1003064
- Wang W, Li H, Lin X et al (2017) Identification and characterization of miRNAs involved in adventitious branches formation of Gracilaria lichenoides in vitro. J Appl Phycol 29:607–615. https://doi.org/10.1007/s10811-016-0930-4
- Wang X, Dong H-P, Wei W et al (2018) Dual expression of plastidial GPAT1 and LPAT1 regulates triacylglycerol production and the fatty acid profile in Phaeodactylum tricornutum. Biotechnol Biofuels 11:318. https://doi.org/10.1186/s13068-018-1317-3
- Yang H, Mao YX, Kong FN et al (2011) Profiling of the transcriptome of Porphyra yezoensis with Solexa sequencing technology. Chin Sci Bull 56:2119–2130. https://doi.org/10.1007/s11434-011-4546-4
- Ye J, Fang L, Zheng H et al (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34:293–297. https://doi.org/10.1093/nar/gkl031
- Yu WL, Ansari W, Schoepp NG et al (2011) Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb Cell Fact 10:91
- Yu M, Yang S, Lin X (2016) De-novo assembly and characterization of Chlorella minutissima UTEX2341 transcriptome by paired-end sequencing and the identification of genes related to the biosynthesis of lipids for biodiesel. Mar Genomics 25:69–74. https://doi.org/10.1016/j.margen.2015.11.005
- Zheng M, Tian J, Yang G et al (2013) Transcriptome sequencing, annotation and expression analysis of Nannochloropsis sp. at different growth phases. Gene 523:117–121. https://doi.org/10.1016/j.gene.2013.04.005
Author Information
Department of Botany, University of Delhi, Delhi, India