Developing mathematical model for diurnal variations of photosynthetic responses in Jatropha curcas L. under soil flooding
Research Articles | Published: 08 February, 2021
First Page: 212
Last Page: 219
Views: 3696
Keywords: Photosynthetic CO2 assimilation rate, Jatropha curcas L., Stomatal conductance, Soil flooding, Transpiration
Abstract
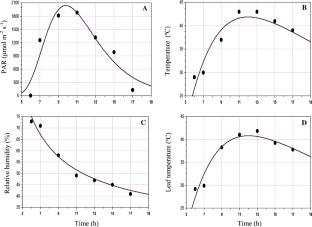
The effect of soil flooding on diurnal variations of photosynthetic responses were analysed in leaves of Jatropha curcas L. plant under open environmental variables. Due to decrease in diurnal stomatal opening and stomatal limitation, the photosynthesis was down regulated. Decrease in carbon uptake of flooded seedlings decreased stomatal opening and limitation. A hypothesis was developed to explain the diurnal variation of photosynthetic responses. From the hypothesized differential equation and prevailing boundary conditions a model was derived to explain the diurnal variations of photosynthetic response under control as well as flooded conditions. Numerical integration of derived model gave the cumulative photosynthetic responses at any time.
References
- Abrol IP (1994) Land degradation—a challenge to sustainability. In: Rao DLN, Singh NT, Gupta RK, Tyagi NK (eds) Salinity management for sustainable agriculture. CSSRI, Karnal, VII–VIII
- Achten WMJ, Verchot L, Franken YJ, Mathijs E, Singh VP, Aerts R (2008) Jatropha bio-diesel production and use. Biomass Bioenergy 32:1063–1084
- Achten WMJ, Maes WH, Reubens B, Mathijs E, Singh VP, Verchot L, Muys B (2010) Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass Bioenergy 34:667–676
- Aggarwal PK, Kalra N, Chander S, Pathak H (2006) Info crop: a dynamic simulation model for the assessment of crop yields, losses due to pests, and environmental impact of agro-ecosystems in tropical environments. I. Model description. Agric Syst 89:1–25
- Armstrong W, Drew MC (2002) Root growth and metabolism under oxygen deficiency. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York, pp 729–761
- Baruch Z (1994) Responses to drought and flooding in tropical forage grasses. II. Leaf water potential, photosynthesis rate and alcohol dehydrogenase activity. Plant Soil 164:97–105
- Boyer JS (1982) Plant productivity and environment. Science 218:443–448
- Bradford KJ, Hsiao TC (1982) Stomatal behaviour and water relations of waterlogged tomato plants. Plant Physiol 70:1508–1513
- Bradford KJ, Yang SF (1981) Xylem transport of 1-aminocyclopropane- 1-carboxylic acid an ethylene precursorr in waterlogged tomato plants. Plant Physiol 65:322–326
- Dat JF, Capellin N, Flozer H, Bourgeade P, Badot M (2004) Sensing and signaling during plant flooding. Plant Physiol Biochem 42:273–282
- de Souza TC, Magalhaes PC, Pereira FJ, de Castro EM, Parentoni SN (2011) Morpho-physiology and maize grain yield under periodic soil flooding in successive selection cycles. Acta Physiol Plantarum 33:1877–1885
- Else MA, Janowiak F, Atkinson CJ, Jackson MB (2009) Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann Bot 103:313–323
- Francis G, Edinger R, Becker KA (2005) Concept for simultaneous wasteland reclamation, fuel production, and socio-economic development in degraded areas in India: need, potential and perspectives of Jatropha plantations. Natural Res Forum 29:12–24
- GEXSI (2008) Global market study on Jatropha-final report. GEXSI LLP, Berlin. http://tinyurl.com/cnyn44
- Gravatt DA, Kirby CJ (1998) Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiol 18:411–417
- Holmberg N, Lilius G, Bailey JE, Bulow L (1997) Transgenic tobacco expressing Vitreoscilla haemoglobin exhibits enhanced growth and altered metabolite production. Nat Biotech 15:244–247
- Islam MR, Hamid A, Karim MA, Haque MM, Khaliq QA, Ahmed JU (2008) Gas exchanges and yield responses of mungbean (Vigna radiate L. Wilczek) genotypes differing in flooding tolerance. Acta Physiol Plantarum 30:697–707
- Jackson MB (2004) The impact of flooding stress on plants and crops. http://www.plantstress.com/articles/index.asp
- Jackson MB (2006) Plant survival in wet environments: resilience and escape mediated by shoot systems. In: Bobbink R, Beltman B, under normal and soil flooding conditions. Verhoeven JTA, Whigham DE (eds) Wetlands: functioning, biodiversity, conservation and restoration, vol 191. Ecological studies, Springer, Berlin, pp 15–36
- Jackson MB (2008) Ethylene-promoted elongation: an adaptation to submergence stress. Ann Bot 101:229–248
- Jackson MB, Davies WJ, Else MA (1996) Pressure-flow relationships, xylem solutes and root hydraulic conductance in flooded tomato plants. Ann Bot 77:17–24
- Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol 1:1–29
- Li M, Hou G, Yang D, Ding G, Li W (2010) Photosynthetic traits of Carex cinerascens in flooded and nonflooded conditions. Photosynthetica 48:370–376
- Lytle CM, Lytle FW, Smith BN (1996) Use of XAS to determine the chemical speciation of bioaccumulated manganese in Potamogeton pectinatus. J Environ Qual 25:311–316
- Mielke MS, de Ameida AAF, Gomes FP, Aguilar MAG, Mangabeira PAO (2003) Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ Exp Bot 50:221–231
- Nery AR, Rodrigues LN, Silva MBR, Fernandes PD, Chaves LHG, Dantas-Neto J (2009) Crescimento do pinha˜ omanso irrigado com a´guas salinas em ambiente protegido. Rev Bras de Eng Agrícola e Ambiental 13:551–558
- Openshaw KA (2000) Review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenergy 19:1–15
- Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312
- Pociecha E, Koscielniak J, Filek W (2008) Effects of root flooding and stage of development on the growth and photosynthesis of field bean (Vicia faba L. minor). Acta Physiol Plantarum 30:529–535
- Pompelli MF, Antunes WC, Ferreira DTRG, Cavalcante PGS, Wanderley-Filho HCLL, Endres L (2012) Allometric models for non-destructive leaf area estimation of Jatropha curcas. Biomass Bioenergy 36:77–85
- Reubens B, Achten WMJ, Maes WH, Danjon F, Aerts R, Poesen J (2011) More than biofuel? Jatropha curcas root system symmetry and potential for soil erosion control. J Arid Environ 75:201–205
- Shabala S (2011) Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol 190:289–298
- Tan S, Zhu M, Zhang Q (2010) Physiological responses of bermudagrass (Cynodon dactylon) to submergence. Acta Physiol Plantarum 32:133–140
- Verma KK, Singh M, Verma CL (2012) Developing a mathematical model for variation of physiological responses of Jatropha curcas leaves depending on leaf positions under soil flooding. Acta Physiol Plantarum 34:1435–1443
- Verma KK, Singh M, Gupta RK, Verma CL (2014) Photosynthetic gas exchange, chlorophyll fluorescence, antioxidant enzymes and growth responses of Jatropha curcas L. during soil flooding. Turk J Bot 48:130–140
- Visser EJW, Voesenek LACJ (2004) Acclimation to soil flooding—sensing and signal-transduction. Plant Soil 254:197–214
- Yordanova RY, Popova LP (2007) Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol Plantarum 29:535–541
- Zahawi RA (2005) Establishment and growth of living fence species: an overlooked tool for the restoration of degraded areas in the tropics. Restor Ecol 13:92–102
- Zhou MZ (2010) Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer-Verlag, Heidelberg, pp 267–285
Author Information
Department of Botany, University of Lucknow, Lucknow, India