Development of efficient micropropagation, assessment of genetic fidelity and biochemical fidelity in Curcuma longa L.
*Article not assigned to an issue yet
Dhondi Prasanna, Kota Srinivas, Rohela Gulab Khan, Kasula Kiranmayee
Research Articles | Published: 19 June, 2025
First Page: 0
Last Page: 0
Views: 900
Keywords: Curcumin, HPLC, In vitro regeneration, ISSR, PGR, SCoT
Abstract
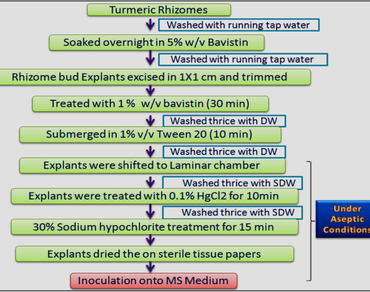
Turmeric (Curcuma longa L.), a key source of curcumin, is highly demanded in food, pharmaceuticals, cosmetics, and traditional medicine industries. India, a leading producer, has benefited significantly from this demand, boosting its spice exports and ranking third globally among spice-exporting nations. To meet the growing demand and enhance turmeric production, prime cultivars like DR (DR) and BSR require efficient mass propagation methods ensuring genetic and curcumin consistency. In this context, a reliable and effective in vitro regeneration protocol was developed from unsprouted rhizome buds of these prime cultivars. On MS media with single cytokinin and auxin, dormancy breakage with highest mean shoot number of 2.40 ± 0.33 per explant was obtained in cv. DR on 0.2 mg L−1 of Kinetin (Kn) and 0.2 mg L−1 α-Naphthalene Acetic Acid (NAA) and mean shoot number of 4.90 ± 0.24 per explant was induced in cv. BSR on 0.7 mg L−1 Kn and 0.2 mg L−1 NAA. Upon sub-culturing of induced shoots on various combinations of double cytokinin with auxin supplemented media, higher frequency of shoot induction (37.30 ± 0.61 shoots in cv. DR and 26.90 ± 0.76 shoots cv. BSR), shoot elongation and rooting was achieved on 6-Benzyl Amino Purine (BAP) (2.0 mg L−1), Thidiazuron (TDZ) (2.0 mg L−1), and (NAA) (2.0 mg L−1) supplemented MS media. In vitro raised plantlets were acclimatized in field conditions with 70% survival rate. The genetic homogeneity of in vitro micropropagated plantlets were confirmed by using Inter Simple Sequence Repeats (ISSR) and Start Codon Targeted (SCoT) markers. Further, the curcumin content quantified through High Pressure Liquid Chromatography (HPLC) technique has showed biochemical fidelity of in vitro and ex vitro grown plants. In conclusion, the developed protocol proves invaluable for the micropropagation of true-to-type turmeric plants of DR and BSR cultivars.
References
Aarthi S, Suresh J, Prasath D (2018) Morphological characterization of Indian turmeric (Curcuma longa L.) genotypes using DUS descriptor. J Plant Crops 46(3):173–179
Aarthi S, Suresh J, Leela NK, Prasath D (2020) Multi environment testing reveals genotype-environment interaction for curcuminoids in turmeric (Curcuma longa L.). Ind Crop Prod 145:112090
Ahmad N, Faisal M, Ahmad A, Alatar AA, Qahtan AA, Alok A (2022) Thidiazuron induced in vitro clonal propagation of Lagerstroemia speciosa (L.) Pers.—an important avenue tree. Horticulturae 8(5):359
Ali A, Munawar A, Siddiqui F (2004) In vitro propagation of turmeric (Curcuma longa L.). In J Biol Biotechnol 1:511–518
Arghya G, Padma C, Parthadeb G (2013) A protocol for rapid propagation of genetically true to type Indian turmeric (Curcuma longa L.) through in vitro culture technique. Adv Appl Sci Res 4:39–45
Badhepuri MK, Beeravelli PR, Arolla RG, Jogam P, Rohela GK, Singisala NR (2024) Micropropagation and genetic fidelity analysis using SCoT and ISSR markers in Muehlenbeckia platyclada (F. Muel.l) meisn. Plant Cell Tissue Organ Cult (PCTOC) 157(3):51
Bandara MMNT, Dahanayake N, Subasinghe S, Perera PCD (2021) A review on in vitro propagation of turmeric (Curcuma longa Ln.). J Univ Ruhuna 9:39–46
Bansal S, Sharma MK, Joshi P, Malhotra EV, Latha M, Malik SK (2024) An efficient direct organogenesis protocol for in vitro clonal propagation of Rubiacordifolia L. Ind Crop Prod 208:117856
Behera K, Pani D, Sahoo S (2010) Effect of plant growth regulator on in vitro multiplication of turmeric (Curcuma longa L. cv. Ranga). Int J Biol Technol 1:16–23
Behera S, Monalisa K, Meher RK, Mohapatra S, Madkami SK, Das PK, Naik PK, Naik SK (2022) Phytochemical fidelity and therapeutic activity of micropropagated Curcuma amada Roxb: a valuable medicinal herb. Ind Crops Prod 176(3):114401
Behera S, Kar SK, Monalisa K, Mohapatra S, Meher RK, Barik DP, Panda PC, Naik PK, Naik SK (2023) Assessment of genetic, biochemical fidelity, and therapeutic activity of in vitro regenerated Hedychiumcoronarium. In Vitro Cell Dev Biol Plant 59(3):602–620
Bharalee R, Das A, Kalita MC (2005) In vitro clonal propagation of Curcuma caesia Roxb and Curcuma zedoaria Rosc from rhizome bud explants. J Plant Biochem 14:61–63
Collard BC, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27:86–93
Dash U, Gupta B, Bhardwaj DR, Sharma P, Kumar D, Chauhan A, Das J (2024) Tree spacings and nutrient sources effect on turmeric yield, quality, bio-economics and soil fertility in a poplar-based agroforestry system in Indian Himalayas. Agro Syst 98:911–931
Dhondi P, Uzma J, Talla SK, Kasula K (2022) Evaluating the role of antioxidant defense system upon exposure to high temperature in different cultivars of Curcuma longa L. Int J Biosci Biochem 4(1):01–05
Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, Fuloria NK (2022) A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front Pharmacol 13:820806
Goyal AK, Ganguly K, Mishra T, Sen A (2010) In vitro multiplication of Curcuma longa Linn.—an important medicinal zingiber. Plant Sci 4:21–24
He R, Gang DR (2013) Somatic embryogenesis and Agrobacterium-mediated transformation of turmeric (Curcuma longa L.). Plant Cell Tiss Org Cult 116(3):333–342
Kadiyala MDM, Gummadi S, Irshad MA, Palanisamy R, Gumma MK, Whitbread A (2021) Assessment of climate change and vulnerability in Indian state of Telangana for better agricultural planning. Theor Appl Clim 143:309–325
Kar B, Kuanar A, Singh S, Mohanty S, Joshi RK, Subudhi E, Nayak S (2014) In vitro induction, screening and detection of high essential oil yielding somaclones in turmeric (Curcuma longa L.). Plant Growth Regul 72:59–66
Koppula T, Sandhya D, Rohela GK, Kommidi S, Mohammed M (2023) Micropropagation and assessment of genetic homogeneity of regenerants by ISSR and SCoT markers in Solena amplexicaulis (Lam.) Gandhi—a threatened medicinal cucurbit. In Vitro Cell Dev Biol Plant 59(6):724–733
Kulyal P, Kuchibhatla LN, Maheshwari KU, Babu KN, Tetali SD, Raghavendra AS (2016) Highly sensitive HPLC method for estimation of total or individual curcuminoids in Curcuma cultivars and commercial turmeric powders. Curr Sci 111:1816–1824
Kulyal P, Acharya S, Ankari AB, Kokkiripati PK, Tetali SD, Raghavendra AS (2021) Variable secondary metabolite profiles across cultivars of Curcuma longa L. and C. aromatic Salisb. Front Pharmacol 12:659546
Murray M, Thompson W (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4326
Nair KP, Nair KP (2019) Diseases of turmeric. Turmeric (Curcuma longa L.) and ginger (Zingiber officinale Rosc.)—world’s invaluable medicinal spices. The agronomy and economy of turmeric and ginger. Springer International Publishing, Cham, pp 568, 151–568171
Nayak S (2000) In vitro multiplication and microrhizome induction in Curcuma aromatic Salisb. Plant Growth Regul 32:41–47
Pai SR, Desai NS (2018) Effect of TDZ on various plant cultures. Thidiazuron: from urea derivative to plant growth regulator. Springer, Singapore, pp 471, 439–471454
Panda MK, Mohanty S, Subudhi E, Acharya L, Nayak S (2007) Assessment of genetic stability of micropropagated plants of Curcuma longa L. by cytophotometry and RAPD analyses. Int J Integr Biol 1:189–195
Pasternak TP, Steinmacher D (2024) Plant growth regulation in cell and tissue culture in vitro. Plants 13(2):327
Pittampalli B, Jogam P, Thampu RK, Abbagani S, Peddaboina V (2021) High-frequency plant regeneration and genetic homogeneity assessment of regenerants by molecular markers in turmeric (Curcuma longa L.). In Vitro Cell Dev Biol Plant 58:169–180
Prakash S, Elangomathavan R, Seshadri S, Kathiravan K, Ignacimuthu S (2004) Efficient regeneration of Curcuma amada Roxb. plantlets from rhizome and leaf sheath explants. Plant Cell Tiss Org Cult 78(2):159–165
Prasath D, Kandiannan K, Leela NK, Aarthi S, Sasikumar B, Babu KN (2018) Turmeric: botany and production practices. Hortic Rev 46:99–184
Prathanturarug S, Soonthornchareonnon N, Chuakul W, Phaidee Y, Saralamp P (2003) High-frequency shoot multiplication in Curcuma longa L. using thidiazuron. Plant Cell Rep 21:1054–1059
Rohela GK, Jogam P, Mir MY, Shabnam AA, Shukla P, Abbagani S, Kamili AN (2020) Indirect regeneration and genetic fidelity analysis of acclimated plantlets through SCoT and ISSR markers in Morusalba L. cv. Chinese white. Biotechnol Rep 25:e00417
Rohela GK, Jogam P, Saini P, Sandhya D, Peddaboina V, Shekhawat MS (2022) Assessing the genetic stability of in vitro raised plants. Commercial scale tissue culture for horticulture and plantation crops. Springer, Singapore, pp 245–276
Sahoo S, Singh S, Sahoo A, Sahoo BC, Jena S, Kar B, Nayak S (2020) Molecular and phytochemical stability of long term micropropagated greater galanga (Alpinia galanga) revealed suitable for industrial applications. Ind Crop Prod 148:112274
Salvi ND, George L, Eapen S (2000) Direct regeneration of shoots from immature inflorescence cultures of turmeric. Plant Cell Tiss Org Cult 62:235–238
Salvi ND, George L, Eapen S (2002) Micropropation and field evaluation of microprotagated plants of turmeric. Plant Cell Tiss Org Cult 68:143–151
Saras T (2023) Turmeric unveiled: exploring the golden spice’s health benefits and culinary marvels. Tiram Media, Central Java First Printing, Agustus, pp. 78, 25–30
Shahinozzaman M, Ferdous M, Faruq M, Azad M, Amin M (2013) Micropropagation of black turmeric (Curcuma caesia Roxb.) through in vitro culture of rhizome bud explants. J Cent Euro Agric 14:110–115
Sharma S, Mamta S, Singh A, Vishal R, Lal A (2012) In vitro protocol standardization for turmeric nultiplicationin Jammu Division. Asian J Hortic 7:151–153
Sinchana N, Kattimani K, Prabhuling G, Sudesh K, Jagadeesha N (2020) Standardization of tissue culture protocol for turmeric (Curcuma longa L.) cv. Salem. Int J Chem Stud 8:2721–2726
Singh S, Kuanar A, Mohanty S, Subudhi E, Nayak S (2010) Evaluation of phytomedicinal yield potential and molecular profiling of micropropagated and conventionally grown turmeric (Curcuma longa L.). Plant Cell Tiss Org Cult 104:263–269
Skiba MB, Luis PB, Alfafara C, Billheimer D, Schneider C, Funk JL (2018) Curcuminoid content and safety related markers of quality of turmeric dietary supplements sold in an urban retail marketplace in the United States. Mol Nut Food Res 62(14):1800143
Soman P (2019) Studies on optimization of fertigation levels to maximize the vigour and rhizome yield of turmeric varieties. Int J Agric Sci 11:8891–8894
SoundarRaju C, Aslam A, Shajahan A (2015) High-efficiency direct somatic embryogenesis and plant regeneration from leaf base explants of turmeric (Curcuma longa L.). Plant Cell Tiss Org Cult 122:79–87
Srirat P, Sirisansaneeyakul S, Parakulsuksatid P, Prammanee S, Vanichsriratana W. In vitro shoot propagation of Curcuma longa L. from rhizome bud explants. In: The international conference on fermentation technology for value agricultural products. 2008. pp. 1–5.
Sujatha D, Chithakari R, Raghuvardhan L, Prasad B, Khan G (2013) In vitro plantlet regeneration and genetic transformation of sponge gourd (Luffa cylindrica L.). Afr J Plant Sci 7(6):244–252
Taghavi T, Rahemi A, Rafie R, Kering MK (2021) Optimizing turmeric tissue culture, testing different media and a plant growth regulator matrix. HortTechnology 31:692–704
Tyagi RK, Yusuf A, Dua P, Agrawal A (2004) In vitro plant regeneration and genotype conservation of eight wild species of curcuma. Biol Plant 48:129–132
Ugochukwu SC, Bob SE, Ozioma O, Odii EB, Ijeoma IC, Olanike O (2013) Shoot proliferation of in vitro turmeric (Curcuma longa L.) affected by different concentrations of benzylaminopurine (BAP). World J Agric Sci 9:227–230
Upendri H, Seranb TH (2020) Effect of explants and low cost medium on morphogenic response of aerial stem and rhizome bud explants of turmeric (Curcuma longa L.) for plant regeneration. J Hortic Plant Res 1:15–24
Vaiserman AM, Lushchak V, Zayachkivska A, Koliada A (2023) Curcumin. Anti-aging pharmacology. Academic Press, Cambridge, pp 153–176
Author Information
Department of Biotechnology, Telangana University, Nizamabad, India