Different spectral qualities do not influence the in vitro and ex vitro survival of Epidendrum denticulatum Barb. Rod.: a Brazilian orchid
Cabral Nadhine Nostrani, Pescador Rosete, Pinheiro Marcos Vinícius Marques, Ornellas Thiago Sanches, Rizzolo Rafaela Gadret, Bordallo Samya Uchôa, Guterres Suelen Martinez, Gris Tainara, Pinheiro Marcos Vinícius Marques, Ornellas Thiago Sanches
Research Articles | Published: 09 August, 2022
First Page: 427
Last Page: 441
Views: 3813
Keywords: Light spectrums, LED lights, Brazilian orchids, Epidendrum
Abstract
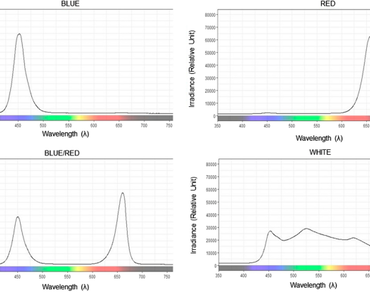
Epidendrum denticulatum is a Brazilian orchid species with few studies related to its physiology during the acclimatization process. This work aimed to understand and elucidate how this species respond to LED lights at the end of in vitro culture and during the ex vitro acclimatization. 14 weeks old plants of the species, originated from seeds cultivated in MS medium, were subjected to blue (B), red (R), and white (W) monochromatic LEDs along with blue/red (B/R) combination for 90 days. Plants were evaluated on the 90th day of in vitro culture under LED lights and the 45th day of acclimatization under natural light. Morphometric parameters were analyzed which included dry and fresh mass, number of leaves, plant height; photosynthetic pigments i.e., chlorophyll, carotenoid, and anthocyanin content along with physiological parameters such as chlorophyll a fluorescence and gas exchange. LED lights did not influenced the survival of the species but some morphological, biochemical and physiological parameters were influenced by the wavelength to which the plants were exposed. Higher fresh mass and dry mass under in vitro conditions were obtained with W light. B light provided a higher value of anthocyanins in in vitro and a lower rate of net photosynthesis under ex vitro conditions. Higher total chlorophyll values were obtained under B/R light. B and B/R wavelengths induced higher Fv/Fm values. 100% survival of the plants were obtained, regardless of the LED applied. W LED light can be indicated for the cultivation of this species during the phases in which the plants were studied, since under this spectral quality the plants developed well and showed good development parameters, in addition they are more available in the market for cheaper rates. This study contributes to a better understanding of the morpho-physiology of Brazilian orchid and is the first study dedicated to the study of the acclimatization of the species E. denticulatum.
References
Agarwal A, Gupta SD (2016) Impact of light-emitting diodes (LEDs) and its potential on plant growth and development in controlled-environment plant production system. Curr Biotechnol 5:28–43. https://doi.org/10.2174/2211550104666151006001126
Ahmed HA, Yu-Xin T, Qi-Chang Y (2020) Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: a review. S Afr J Bot 130:75–89. https://doi.org/10.1016/j.sajb.2019.12.018
Almeida AM, Figueiredo RA (2003) Ants visit nectaries of Epidendrum denticulatum (Orchidaceae) in a Brazilian rainforest: effects on herbivory and pollination. Braz J Biol 63:551–558. https://doi.org/10.1590/S1519-69842003000400002
Araújo DX, Rocha TT, de Carvalho AA, Bertolucci SKV, Medeiros APR, Ribeiro FNS, Barbosa SM, Pinto JEBP (2021) Photon flux density and wavelength influence on growth, photosynthetic pigments and volatile organic compound accumulation in Aeollanthus suaveolens (Catinga-de-mulata) under in vitro conditions. Ind Crops Prod 168:113597. https://doi.org/10.1016/j.indcrop.2021.113597
Arena C, Tsonev T, Doneva DV, De Micco M, Michelozzi C, Brunetti M, Centritto S, Fineschi V, Velikova F (2016) The efect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ Exp Bot 16:8472. https://doi.org/10.1016/j.envexpbot.2016.05.014
Badr A, Angers P, Desjardins Y (2011) Metabolic proofing of photoautotrophic and photomixotrophic potato plantlets (Solanum tuberosum) provides new insights into acclimatization. PCTOC 107:13–24. https://doi.org/10.1007/s11240-011-9951-5
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621. https://doi.org/10.1093/jxb/erh196
Batista DS, Castro KM, Silva AR, Teixeira ML, Sales TA, Soares LI, Cardoso MG, Santos MO, Viccini LF, Otoni WC (2016) Light quality affects in vitro growth and essential oil profile in Lippia alba (Verbenaceae). In Vitro Cell Dev Biol Plant. https://doi.org/10.1007/s11627-016-9761-x
Bello-Bello JJ, Martínez-Estrada E, Caamal-Velázquez JH, Morales-Ramos V (2016) Effect of LED light quality on in vitro shoot proliferation and growth of vanilla (Vanilla planifolia Andrews). Afr J Biotechnol 15:272–277. https://doi.org/10.5897/AJB2015.14662
Bello-Bello JJ, Perez-Sato JA, Cruz-Cruz CA, Martinez-Estrada E (2017) Light-emitting diodes: progress in plant micropropagation. INTECH 6:93–103. https://doi.org/10.5772/67913
Berkovich YuA, Konovalova IO, Smolyanina SO, Erokhin AN, Avercheva OV, Bassarskaya EM, Kochetova GV, Zhigalova TV, Yakovleva OS, Tarakanov IG (2017) LED crop illumination inside space greenhouses. REACH Rev Hum Space Exploration 6:11–24. https://doi.org/10.1016/j.reach.2017.06.001
Billore V, Jain M, Suprasanna P (2017) Monochromic radiation through light-emitting diode (LED) positively augments in vitro shoot regeneration in Orchid (Dendrobium sonia). Can J Biotech 1:50–58. https://doi.org/10.24870/cjb.2017-000106
Bjorkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Zeigler H (eds) Physiological Plant Ecology I, Ency in Plant Physiology, NS, 12A. Springer, New York. https://doi.org/10.1007/978-3-642-68090-8_4
Bolhar-Nordenkampf HR, Long SP, Baker NR, Oquist G, Schreiber U, Lechner EG (1989) Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Funct Ecol 3:497–514. https://doi.org/10.2307/2389624
Carvalho AAd, Bertolucci SKV, Honorato AdC, Rocha TT, Silva ST, Pinto JEBP (2020) Influence of light spectra and elicitors on growth and ascaridole content using in vitro cultures of Dysphania ambrosioides L. Plant Cell Tissue Organ Cult 143:277–290. https://doi.org/10.1007/s11240-020-01892-5
Centofante A (2020) Light quality on the morphoanatomy and physiology of Campomanesia pubescens (DC.) O. Berg. seedlings. Sci Hortic 259:108765. https://doi.org/10.1016/j.scienta.2019.108765
Choi HG, Moon BY, Kang NJ (2015) Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci Hortic 189:22–31. https://doi.org/10.1016/j.scienta.2015.03.022
Cope KR, Bugbee B (2013) Spectral effects of three types of white light-emitting diodes on plant growth and development: absolute versus relative amounts of blue light. HortScience 48:504–509. https://doi.org/10.21273/HORTSCI.48.4.504
Dewez D, Perreault F (2012) Effect of the anthocyanic epidermal layer on Photosystem II and I energy dissipation processes in Tradescantia pallida (Rose) Hunt. Acta Physiol Plant 35:463–472. https://doi.org/10.1007/s11738-012-1089-5
Dos Santos CM, Verissimo V, Wanderley Filho HCL, Ferreira VM, Cavalcante PGS, Rolim EV, Endres L (2013) Seasonal variations of photosynthesis gas exchange, quantum efficiency of photosystem II and biochemical responses of Jatropha curcas L. grow in semi-humid and semi-arid areas subject to water stress. Ind Crops Prod 41:203–213. https://doi.org/10.1590/0001-3765201720160729
Dou H, Niu G, Gu M, Masabni JG (2017) Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 3:36. https://doi.org/10.3390/horticulturae3020036
Furuya M (1993) Phytochromes: their molecular species, gene families, and functions. Annu Rev Plant Physiol Plant Mol Biol 44:617–645. https://doi.org/10.1146/annurev.pp.44.060193.003153
Govaerts R, Bernet P, Kratochvil K, Gerlach G, Carr G, Alrich P, Pridgeon AM, Pfahl J, Campacci MA, Baptista DH, Tigges H, Shaw J, Cribb P, George A, Kreuz K, Wood JC (2020) World Checklist of Orchidaceae. Facilitated by the Royal Botanic Gardens, Kew. http://wcsp.science.kew.org/. Accessed 01 July 2021
Gupta SD, Jatothu B (2013) Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol Rep 7:211–220. https://doi.org/10.1007/s11816-013-0277-0
Hágsater E, Soto-Arenas MA (2005) Epidendrum. In: Pridgeon AM, Cribb PJ, Chase MW, e Rasmussen FN (eds) Genera Orchidacearum: 4. Epidendroideae (part one), vol 4. Oxford University Press, Oxford, pp 236–251
Hamdani S, Khan N, Perveen S, Qu M, Jiang J, Govindjee Z-G (2019) Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth Res 139:107–121. https://doi.org/10.1007/s11120-018-0589-6
Heo J, Lee C, Chakrabarty D, Paek K (2002) Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul 38:225–230. https://doi.org/10.1023/A:1021523832488
Hernández R, Kubota C (2016) Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ Exp Bot 121:66–74. https://doi.org/10.3389/fpls.2021.709313
Hsie BS, Bueno AIS, Bertolucci SKV, Carvalho AA, Cunha SHB, Martins ER, Pinto JEBPL (2019) Study of the influence of wavelengths and intensities of LEDs on the growth, photosynthetic pigment, and volatile compounds production of Lippia rotundifolia Cham in vitro. J Photochem Photobiol Biol 198:111577. https://doi.org/10.1016/j.jphotobiol.2019.111577
Huché-Thélier L, Crespel L, Gourrierec JL, Morel P, Sakr S, Leduc N (2016) Light signaling and plant responses to blue and UV radiations—perspectives for applications in horticulture. Environ Exp Bot 121:22–38. https://doi.org/10.1016/j.envexpbot.2015.06.009
Hung CD, Hong CH, Kim SK, Lee K-H, Park J-Y, Nam M-W, Choi D-H, Lee H-I (2016) LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol Plant 38:152. https://doi.org/10.1007/s11738-016-2164-0
Johkan M, Shoji K, Goto F, Hashida S, Yoshihara T (2010) Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 45:1809–1814. https://doi.org/10.21273/HORTSCI.45.12.1809
Jones MA (2018) Using light to improve commercial value. Hortic Res 5:47. https://doi.org/10.1038/s41438-018-0049-7
Juras MCR, Purgatto E, Ferreira WM, Suzuki RM (2020) Direct organogenesis and ethylene regulators in the cloning of Epidendrum denticulatum (Orchidaceae). S Afr J Bot 131:374–379. https://doi.org/10.1016/j.sajb.2020.03.010
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev KI, Brestic M, Bussotti F, Calatayud A, Dabrowski P, Elsheery NI, Ferroni L, Guidi L, Hogewoning SW, Jajoo A, Misra AN, Nebauer SG, Pancaldi S, Penella C, Poli DB, Pollastrini M, Romanowska-Duda ZB, Rutkowska B, Seródio J, Suresh K, Szulc W, Tambussi E, Yanniccari M, Zivcak M (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122:121–158. https://doi.org/10.1007/s11120-014-0024-6
Ko SS, Jhong CM, Shih MC (2020) Blue light acclimation reduces the photoinhibition of Phalaenopsis aphrodite (Moth Orchid). Int J Mol Sci 21:61–67. https://doi.org/10.3390/ijms21176167
Kok T, Irawati F (2020) Effect of three LED lights on the biomass production and copper remediation by shoot cultures of Musa paradisiaca. Heliyon 6:e04981. https://doi.org/10.1016/j.heliyon.2020.e04981
Kozai T (2010) Photoautotrophic micropropagation—environmental control for promoting photosynthesis. Propag Ornam Plants 10:188–204
Landi M, Zivcak M, Sytar O, Brestic M, Allakhverdiev SI (2020) Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: a review. Biochim Biophys Acta Bioenerg 1861:131–148. https://doi.org/10.1016/j.bbabio.2019.148131
Lazzarini LES, Pacheco FV, Silva ST, Coelho AD, Medeiros APR, Bertolucci SKV, Pinto JEBP, Soares JDR (2017) Use of light-emitting diode (LED) in the physiology of cultivated plants—review. Sci Agrar Parana 16:137–144
Lian TT, Cha SY, Moe MM, Kim YJ, Bang KS (2019) Effects of different colored LEDs on the enhancement of biologically active ingredients in callus cultures of Gynura procumbens (Lour.) Merr. Molecules 24:4336. https://doi.org/10.3390/molecules24234336
Lin Y, Li J, Li B, He T, Chun Z (2011) Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult 105:329–335. https://doi.org/10.1007/s11240-010-9871-9
Liu M, Xu Z, Guo S, Tang C, Liu X, Jao X (2014) Evaluation of leaf morphology, structure and biochemical substance of balloon fower (Platycodon grandiforum (Jacq.) A. DC.) plantlets in vitro under diferent light spectra. Sci Hortic 174:112–118. https://doi.org/10.1016/j.scienta.2014.05.006
Maiza R, Kurnia D (2019) The influence of light wavelengths toward the growth of Brassica rapa L. J Phys Conf Ser 1245:012089. https://doi.org/10.1088/1742-6596/1245/1/012089
Manivannan A, Soundararajan P, Halimah N, Ko CH, Jeong BR (2015) Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic Environ Biotechnol 56:105–113. https://doi.org/10.1007/s13580-015-0114-1
Manivannan A, Soundararajan P, Park YG, We H, Kim S, Jeong BR (2017) Blue and red light-emitting diodes improve the growth and physiology of in vitro-grown carnations “Green Beauty” and “Purple Beauty.” Hortic Environ Biotechnol 58:12–20. https://doi.org/10.1007/s13580-017-0051-2
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Mizuno T, Amaki W, Watanabe H (2011) Effects of monochromatic light irradiation by led on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic 907:179–184. https://doi.org/10.17660/ActaHortic.2011.907.25
Morel G, Wetmore RM (1951) Fern callus tissue culture. Am J Bot 38:141–143. https://doi.org/10.2307/2437837
Muneer S, Kim EJ, Park JS, Lee JH (2014) Influence of green, red and blue light-emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int J Mol Sci 15:4657–4670. https://doi.org/10.3390/ijms15034657
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Murray JR, Hackett WP (1991) Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant Physiol 97:343–351. https://doi.org/10.1104/pp.97.1.343
Naznin MT, Lefsrud M, Gravel V, Azad MOK (2019) Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 8:93. https://doi.org/10.3390/plants8040093
Nguyen TKL, Oh M (2021) Physiological and biochemical responses of green and red perilla to LED-based light. J Sci Food Agric 101:240–252. https://doi.org/10.1002/jsfa.10636
Nguyen QT, Xiao Y, Kozai T (2016) Photoautotrophic micropropagation. In: Kozai T, Niu G, Takagaki M (eds) Plant factory: an indoor vertical farming system for efficient quality food production, 2nd edn. Academic Press, New York, pp 271–283
Nishimura T, Ohyama K, Goto E, Inagaki N (2009) Concentrations of perillaldehyde, limonene, and anthocyanin of Perilla plants as affected by light quality under controlled environments. Sci Hortic 122:134–137. https://doi.org/10.1016/j.scienta.2009.03.010
Oliveira VC, Sajo MG (1999) Anatomia foliar de espécies epífitas de Orchidaceae. Rev Bras Bot 22:365–374. https://doi.org/10.1590/S0100-84041999000300003
Olle M, Virsile A (2013) The effects of light-emitting diode lighting on greenhouse plant growth and quality. AFSC 22:223–234. https://doi.org/10.23986/afsci.7897
Ouzounis T, Rosenqvist E, Ottosen CO (2015) Spectral effects of artificial light on plant physiology and secondary metabolism. HortScience 50:1128–1135. https://doi.org/10.21273/HORTSCI.50.8.1128
Palhares Neto L, Souza LM, Morais MB, Albuquerque CC, Camara TR, Ulisses C (2018) Controlling hyperhydricity in micropropagated plants of Lippia grata Schauer (Verbenaceae), a native species of a dry seasonal tropical forest with pharmacological potential. Braz J Bot. https://doi.org/10.1007/s40415-018-0476-6529-438
Paradiso R, Proietti S (2022) Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: the state of the art and the opportunities of modern LED systems. J Plant Growth Regul 41:742–780. https://doi.org/10.1007/s00344-021-10337-y
Pimputkar S, Speck JS, Den Baars SP, Nakamura S (2009) Prospects for LED lighting. Nat Photonics 3:180–182. https://doi.org/10.1038/nphoton.2009.32
Pinheiro F, Barros F (2007) Epidendrum secundum Jacq. e E. denticulatum Barb. Rodr. (Orchidaceae): caracteres úteis para a sua separação. Hoehnea 34:563–570. https://doi.org/10.1590/S2236-89062007000400010
Pinheiro F, Cozzolino S, de Barros F, Gouveia TM, Suzuki RM, Fay MF, Palma-Silva C (2013) Phylogeographic structure and outbreeding depression reveal early stages of reproductive isolation in the neotropical orchid Epidendrum denticulatum. Evolution 67:2024–2039. https://doi.org/10.1111/evo.12085
Ralph PJ, Gademann R (2005) Rapid light curve: a powerful tool for the assessment of photosynthetic activity. Aquat Bot 82:222–237. https://doi.org/10.1016/j.aquabot.2005.02.006
Ramel F, Mialoundama AS, Havaux M (2013) Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J Exp Bot 64:799–805. https://doi.org/10.1093/jxb/ers223
Ramírez-Mosqueda M, Iglesias Andreu LG, Luna-Sánchez IJ (2017) Light quality affects growth and development of in vitro plantlet of Vanilla planifolia Jacks. S Afr J Bot 109:288–329. https://doi.org/10.1016/j.sajb.2017.01.205
Rao MJ, Xu Y, Huang Y, Tang X, Deng X, Xu Q (2019) Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol 19:603. https://doi.org/10.1186/s12870-019-2212-1
Resende CF, Bianchetti RE, Oliveira MA, Braga VF, Peixoto PH (2015) In vitro propagation and acclimatization of Lippia rotundifolia, na endemic species of Brazilian Campos Rupestres. Rev Cien Agron 46:582–589. https://doi.org/10.5935/1806-6690.20150041
Ronquim CC, Prado CHBA, Souza JP (2009) Growth, photosynthesis and leaf water potential in Young plants of Capaifera langsdorffii Desf. (Caesalpiniaceae) under contrasting irradiances. Braz J Plant Physiol 21:197–208. https://doi.org/10.1590/S1677-04202009000300004
Sabzalian MR, Heydarizadeh P, Zahedi M, Boroomand A, Agharokh M, Sahba MR, Schoefs B (2014) High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron Sustain Dev 34:879–886. https://doi.org/10.1007/s13593-014-0209-6
São Leão LC (2012) Estudos reprodutivos em duas espécies simpátricas de Epidendrum L. (Epidendroideae–Orchidaceae) em vegetação de restinga. Dissertation, Universidade Federal do Rio de Janeiro
Shin KS, Murthy HN, Heo JW, Haln EJ, Paek KY (2008) The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol Plant 30:339–343. https://doi.org/10.1007/s11738-007-0128-0
Shin KS, Park SY, Paek KY (2014) Physiological and biochemical changes during acclimatization in a Doritaenopsis hybrid cultivated in different microenvironments in vitro. Environ Exp Bot 100:26–33
Shukla MR, Singh AS, Piunno K, Saxena PK, Jones AMP (2017) Application of 3D printing to prototype and develop novel plant tissue culture systems. Plant Methods 13:6–15. https://doi.org/10.1186/s13007-017-0156-8
Spalholz H, Perkins-Veazie P, Hernandez R (2020) Impact of sun-simulated white light and varied blue: red spectrums on the growth, morphology, development, and phytochemical content of green-and red-leaf lettuce at different growth stages. Sci Hortic 264:109195. https://doi.org/10.1016/j.scienta.2020.109195
Stancato GC, Mazzafera P, Buckeridge MS (2002) Effects of light stress on the growth of the epiphytic orchid Cattleya forbesii Lindl. × Laelia tenebrosa Rolfe. Rev Bras Bot 2:229–235. https://doi.org/10.1590/S0100-84042002000200011
Stancik JF, Goldenberg R, Barros F (2009) O gênero Epidendrum L. (Orchidaceae) no Estado do Paraná, Brasil. Acta Bot Bras 23:864–880. https://doi.org/10.1590/S0102-33062009000300028
StatSoft Inc STATISTICA (2010) Data analysis software system, version 10. https://www.statsoft.com. Accessed July 2021
Tarakanov IG, Kosobryukhov AA, Tovstyko DA, Anisimov AA, Shulgina AA, Sleptsov NN, Kalashnikova EA, Vassilev AV, Kirakosyan RN (2021) Effects of light spectral quality on the micropropagated raspberry plants during ex vitro adaptation. Plants 10:2071. https://doi.org/10.3390/plants10102071
Tester M, Bacic A (2005) Abiotic stress tolerance in grasses. From model plants to crop plants. Plant Physiol 37:791–793. https://doi.org/10.1104/pp.104.900138
Wang J, Lu W, Tong Y, Yang Q (2016) Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front Plant Sci 7:250. https://doi.org/10.3389/fpls.2016.00250
Wellburn AR (1994) Determination of chlorophyll-a and chlorophyll-b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Whitelam G, Halliday K (2007) Light and plant development. Blackwell Publishing, Oxford
Wu MC, Hou CY, Jiang CM, Wang UT, Wang CU, Chen HH, Chang HM (2007) A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem 101:1753–1758. https://doi.org/10.1016/j.foodchem.2006.02.010
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. PCTOC 105:149–158. https://doi.org/10.1007/s11240-010-9863-9
Zhang Y, Jiang L, Li Y, Chen Q, Ye Y, Zhang Y, Luo Y, Sun B, Wang X, Tang H (2018) Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 23:820. https://doi.org/10.3390/molecules23040820
Author Information
Postgraduate Program in Plant Genetic Resources, Center of Agrarian Sciences, Santa Catarina Federal University, Florianópolis, Brazil