Differential Calcium responsiveness in terms of Plant’s Growth and accumulation of Nutrient-Anti nutrient in two Finger Millet Genotypes differing in grain Calcium content using a designed Circulatory Hydroponics system
Naved Akbar, Manoj Singh, Supriya Gupta, K. P. Singh, Anil Kumar
Research Article | Published: 11 January, 2019
First Page: 16
Last Page: 24
Views: 3804
Keywords: Nutrient, Antinutrient, Calcium, Oxalate, Hydroponics.
Abstract
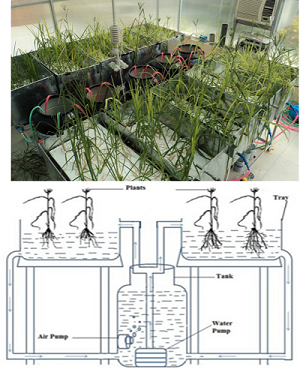
Finger millet (Eleusine coracana) commonly known as ragi is model crop of choice for variety of reasons. These include its high nutraceutical value, excellent storage qualities and most important low input crop. In this study, circulatory hydroponic system was designed and optimized to assess the nutrient responsiveness under the influence of exogenous calcium (Ca) application in finger millet. Impact of different concentrations of exogenous Ca in the form of calcium nitrate on plant’s growth and nutrient-antinutrient changes were determined in grains of two contrasting genotypes GP-1 (low Ca) and GP-45 (high Ca) of finger millet. The plants were supplied regularly with different levels of Ca in Hoagland’s nutrient medium. Up to 10 mM of exogenous supplied Ca concentration, the threshold potential in terms of Ca accumulation as well as different plant’s growth parameters of GP-45 (high Ca) genotype was higher than GP-1 (low Ca); while at 20 mM of Ca concentration, the decline in Ca accumulation and plant’s growth was observed in both the genotypes. The highest Ca accumulation was recorded in GP-45 genotype at 10 mM of Ca concentration. Interestingly, with increasing concentration of exogenous Ca, a higher oxalic acid content in grains was observed in GP-1 while higher content of tartaric acid were observed in GP-45 genotype having high grain Ca. These results revealed that Ca interacts with oxalic acid and other organic acids like tartaric acid which is genotype dependent. Hence, the interaction of Ca with antinutrient(s) might be under control of both genetic and epigenetic factor(s).
References
- Vadivoo AS, Joseph R and Ganesan NM (1998). Genetic variability and diversity for protein and calcium contents in finger millet (Eleusine coracana (L.) Gaertn) in relation to grain color. Plant Foods Hum Nutr 52(4): 353-64.
- Antony U and Chandra TS (1998). Antinutrient reduction and enhancement in protein, starch, and mineral availability in fermented flour of finger millet (Eleusine coracana). Journal of Agric Food Chem 46(7): 2578-2582.
- Ignacimuthu S and Ceasar SA (2012). Development of transgenic finger millet (Eleusine coracana (L.) Gaertn.) resistant to leaf blast disease. J Biosci 37(1):135-147.
- Kumar A, Metwal M, Kaur S, Gupta AK, Puranik S, Singh S, Singh M, Gupta S, Babu BK, Sood S and Yadav R (2016). Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Frontiers Plant Sci 7.
- Panwar P, Nath M, Yadav VK and Kumar A (2010). Comparative evaluation of genetic diversity using RAPD, SSR and cytochrome P450 gene based markers with respect to calcium content in finger millet (Eleusine coracana L. Gaertn.). J Genet 89(2):121-133.
- Singh UM, Chandra M, Shankhdhar SC and Kumar A (2014a). Transcriptome wide identification and validation of calcium sensor gene family in the developing spikes of finger millet genotypes for elucidating its role in grain calcium accumulation. PloS one 9(8):p.e103963.
- Kumar A, Gaur VS, Goel A and Gupta AK (2015). De novo assembly and characterization of developing spikes transcriptome of finger millet (Eleusine coracana): a minor crop having nutraceutical properties. Plant molecular biology reporter. 33(4):905-922.
- Singh UM, Metwal M, Singh M, Taj G and Kumar A (2015). Identification and characterization of calcium transporter gene family in finger millet in relation to grain calcium content. Gene 5661:37-46.
- Singh UM, Pandey D and Kumar A (2014b). Determination of calcium responsiveness towards exogenous application in two genotypes of Eleusine coracana L. differing in their grain calcium content. Acta physiol Plant 36(9):2521-2529.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G and Kovacs CS (2011). The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clinical Endocrin Metabol 96(1):53-58.
- White PJ and Broadley MR (2003). Calcium in plants. Ann Bot 92(4): 487-511.
- Wilson GJ and Stephenson DG (1990). Calcium and strontium activation characteristics of skeletal muscle fibres from the small marsupial Sminthopsis macroura. J Muscle Res Cell Motil 11(1):12-24.
- Singh M, Metwal M, Kumar VA and Kumar A (2016). Identification and molecular characterization of 48 kDa calcium binding protein as calreticulin from finger millet (Eleusine coracana) using peptide mass fingerprinting and transcript profiling. J Sci Food Agric 96(2):672-679.
- Raboy V, Gerbasi PF, Young KA, Stoneberg SD, Pickett SG, Bauman AT, Murthy PP, Sheridan WF and Ertl DS (2000). Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol 124(1):355-368.
- Raboy V (2009). Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci 177(4):281-296.
- Ilarslan H, Palmer R, Imsande J and Horner H (1997). Quantitative determination of calcium oxalate and oxalate in developing seeds of soybean (Leguminosae). Amer J Bot 84(8): 1042-1042.
- Amalraj A and Pius A (2015). Bioavailability of calcium and its absorption inhibitors in raw and cooked green leafy vegetables commonly consumed in India–An in vitro study. Food Chem170:430-436.
- Chakraborty N, Ghosh R, Ghosh S, Narula K, Tayal R, Datta A and Chakraborty S (2013). Reduction of oxalate levels in tomato fruit and consequent metabolic remodeling following overexpression of a fungal oxalate decarboxylase. Plant Physiol 162(1):364-378.
- Ahmad P, Jamsheed S, Hameed A, Rasool S, Sharma I, Azooz MM and Hasanuzzaman M (2014). Drought stress induced oxidative damage and antioxidants in plants. Elsevier, New York. Pp. 345-367
- Hodgkinson A (1977). Oxalic acid metabolism in higher plants. In A Hodgkinson, ed, Oxalic Acid Biology and Medicine Academic Press, New York. Pp 131–158.
- Saito K, Ohmoto J and Kuriha N (1997). Incorporation of 18O into oxalic, L-threonic and L-tartaric acids during cleavage of L-ascorbic and 5-keto-D-gluconic acids in plants. Phytochem 44(5):805-809.
- Loewus FA (1999). Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochem 52(2):193-210.
- Nakata PA and McConn MM (2007). Genetic evidence for differences in the pathways of druse and prismatic calcium oxalate crystal formation in Medicago truncatula. Functional Plant Biol 34(4):332-338.
- Nakata PA (2012). Engineering calcium oxalate crystal formation in Arabidopsis. Plant Cell Physiol 53(7):1275-1282.
- Libert B and Franceschi VR (1987). Oxalate in crop plants. J Agric Food Chem 35(6):926-938.
- Kinzel H and Lechner I (1992). The specific mineral metabolism of selected plant species and its ecological implications. Plant Biol 105(5):355-361.
- Prasad R and Shivay YS (2017). Oxalic acid/oxalates in plants: from self-defence to phytoremediation. Curr Sci 112(8):1665-1667.
- Henriksson E and Henriksson K (2005). Salt stress signalling and the role of calcium in the regulation of the Arabidopsis ATHB7 gene. Plant, Cell Environ 28(2):202-210.
- Aurisano N, Bertani A and Reggiani R (1995). Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant Cell Physiol 36(8):1525-1529.
- Gao H, Chen G, Han L and Lin H (2005). Calcium influence on chilling resistance of grafting eggplant seedlings. Journal of plant nutrition. 27(8):1327-1339.
- Domingues LDS, Ribeiro ND, Andriolo JL, Possobom MTDF and Zemolin AEM (2016). Growth, grain yield and calcium, potassium and magnesium accumulation in common bean plants as related to calcium nutrition. Acta Scient Agron 38(2): 207-217.
- Arteca RN and Arteca JM (2000). A novel method for growing Arabidopsis thaliana plants hydroponically. Physiologia Plantarum 108(2): 188-193.
- Hoagland DR and Arnon DI (1950). The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station 347(2nd edit).
- Islam AKMS, Edwards DG and Asher CJ (1980). pH optima for crop growth. Plant Soil 54(3):339-357.
- Bugbee B (2003). Nutrient management in recirculating hydroponic culture. In South Pacific Soilless Culture Conference-SPSCC 648:99-112).
- Barbeau WE and Hilu KW (1993). Protein, calcium, iron, and amino acid content of selected wild and domesticated cultivars of finger millet. Plant Foods Human Nutr (Formerly Qualitas Plantarum). 43(2):97-104.
- Muñoz-Robredo P, Robledo P, Manríquez D, Molina R and Defilippi BG (2011). Characterization of sugars and organic acids in commercial varieties of table grapes. Chilean J Agric Res 71(3):452.
- George E, Horst WJ and Neumann E (2012). Adaptation of plants to adverse chemical soil conditions. In P. Marschner ed., Marschner’s Mineral Nutrition of Higher Plants, 3rd edition. Academic Press, San Diego. Pp 409-472.
- Islam MN, Maeda H and Kawasaki M (2015). Effect of Calcium Concentration in Growth Medium on Oxalate Content and Evaluation of the Role of Guttation in the Regulation of Oxalate in Eddo. Plant Prod Sci 18(4): 464-470.
- Keates SE, Tarlyn NM, Loewus FA and Franceschi VR (2000). L-Ascorbic acid and L-galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes. Phytochem 53(4):433-440.
Author Information
Department of Molecular Biology & Genetic Engineering, College of Basic Sciences & Humanities, G B Pant University of Agriculture & Technology, Pantnagar-263145, Uttarakhand, India.