Disease responses of different tomato (Solanum lycopersicum L.) cultivars inoculated with culture filtrates of selected fungal pathogens
Research Articles | Published: 21 January, 2020
First Page: 166
Last Page: 171
Views: 3734
Keywords: Culture filtrates, Fungal pathogens, Tomato cultivars, Susceptibility, Resistance
Abstract
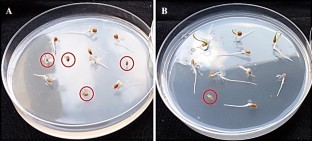
This study evaluates the responses of different tomato cultivars to disease initiation by cell-free culture filtrates (CFCFs) of selected fungal pathogens. Twenty-one days old culture filtrates of four fungal pathogens; Alternaria triglochinicola, Aureobasidium pullulans, Pythium ultimum and Sclerotium rolfsii grown on Potato Sucrose Broth were tested against four tomatoes; Roma Savanna, Tropimech, Riogrande and Roma VF. Seed germination was inhibited by filtrates of Alternaria triglochinicola and Pythium ultimum. Plant heights were lower in Roma Savanna seedlings treated with filtrates of Sclerotium rolfsii (3.27 cm), and Alternaria triglochinicola (3.30 cm); Riogrande seedlings treated with filtrates of Pythium ultimum (2.22 cm) and Alternaria triglochinicola (2.46 cm), compared to controls (3.7 cm and 2.56 cm). Roma Savanna and Riogrande cultivars showed 25% and 50% necrotic responses respectively to Alternaria triglochinicola, while Roma VF showed 50.00% response to Aureobasidium pullulans. Differences in foliar symptoms induced by CFCFs of different fungal pathogens on the studied tomato cultivars were significant (P ≤ 0.05).
References
- Akpaninyang F, Opara E (2017) The influence of toxins in disease symptom initiation in plants: a review. J Agric Sustain 10(1):29–52
- Amusa N (2006) Microbially produced phytotoxins and plant disease management. Afr J Biotechnol 5(5):405–414
- Anand T, Bhaskaran R, Karthikeyan TG, Rajesh M, Senthilraja G (2008) Production of cell wall degrading enzymes and toxins by Colletotrichum capsici and Alternaria alternata causing fruit rot of chillies. J Plant Prot Res 48(4):437–451
- Bem A (2009) Incidence of major fungal diseases of tomato (Lycopersicon esculentum L.) and their evaluation in some tomato growing areas of Benue State. University of Agriculture, Makurdi
- Bhajbhuje M (2015) Response of metabolites from culture filtrates of Alternaria species against Triticum aestivum L. Int J Life Sci 3:55–62
- Criscitiello MF, Dickman MB, Samuel JE, de Figueiredo P (2013) Tripping on acid: trans-kingdom perspectives on biological acids in immunity and pathogenesis. PLoS Pathog 9(7):e1003402
- Dickman MB, Fluhr R (2013) Centrality of host cell death in plant-microbe interactions. Annu Rev Phytopathol 51:543–570
- Dickman M, Mitra A (1992) Arabidopsis thaliana as a model for studying Sclerotinia sclerotiorum pathogenesis. Physiol Mol Plant Pathol 41(4):255–263
- Ellis SD, Boehm MJ, Mitchell TK (2008) Fungal and fungal-like diseases of plants. Fact Sheet (PP401. 07) Agriculture and Natural Resources, The Ohio State University
- Quayyum H, Gijzen M, Traquair J (2003) Purification of a necrosis-inducing, host-specific protein toxin from spore germination fluid of Alternaria panax. Phytopathology 93(3):323–328
- Robeson D, Strobel G (1986) The influence of plant extracts on phytotoxin production and growth rate of Alternaria helianthi. J Phytopathol 117(3):265–269
- Sharma PN (2011) Measurement of disease and assessment of losses. Department of Plant Pathology, CSK HPKV, Palampur
- Stierle AC, Cardellina JH, Strobel GA (1988) Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc Natl Acad Sci 85(21):8008–8011
- Wani A (2011) An overview of the fungal rot of tomato. Mycopathology 9(1):33–38
- Zheng L, Lv R, Huang J, Jiang D, Hsiang T (2010) Isolation, purification, and biological activity of a phytotoxin produced by Stemphylium solani. Plant Dis 94(10):1231–1237
Author Information
Department of Botany, Federal University of Lafia, Lafia, Nigeria