Effect of Biofabricated Silver nanoparticles on Growth parameters in Fenugreek (Trigonella foenum-graecum)
Singh Garvita, Sheokand Anshul, Gupta Ashi, Gupta Himani, Kumar Jay, Soni Renu, Singh Varsha K., Sinha Rajeshwar P., Sinha Rajeshwar P., Sinha Rajeshwar P.
Research Articles | Published: 12 April, 2024
First Page: 1751
Last Page: 1759
Views: 3271
Keywords: Fenugreek, Plant growth, Productivity, Silver nanoparticles, Senescence
Abstract
Plants being the primary producer in the ecosystem and are the most significant source of food and energy for life to sustain; therefore, a better and improved understanding related to the impacts of nanoparticles on plant growth and development needs to be studied. Use of nanotechnology is one of the methods to ensure food security by improving plant growth and productivity. The current study was undertaken to explore interactions of biologically synthesized (from cyanobacterial extract) silver nanoparticles (AgNPs) on growth and development of Trigonella foenum-graecum. Plant responses to AgNPs exposure in features like percentage seed germination, hypocotyl length and root length, chlorophyll content, number of branchlets, number of leaves, number of flowers, pod length, and number of seeds, were examined. The results obtained showed that out of three different concentrations (10 µg/mL, 20 µg/mL and 50 µg/mL) of AgNPs, 10 µg concentration was found to be the most relevant for growth parameters. There was a slight increase in the studied parameters at 20 µg concentration of AgNPs but higher concentration of AgNPs had showed inhibitory effects. The results obtained showed that out of three different concentrations (10 µg/mL, 20 µg/mL and 50 µg/mL) of AgNPs, 10 µg/mL concentration was found to be most relevant in the study of growth parameters. AgNPs at low concentrations (10 µg/mL and 20 µg/mL) showed positive effect on overall development of plant by enhancing the number of branchlets, leaves per plant, number of pods, and seed production. AgNPs caused toxicity to the seedlings at higher concentration and increased exposure time. Other parameters such as early flowering and delayed senescence has also been reported in AgNPs treated plants which can be used for further studies.
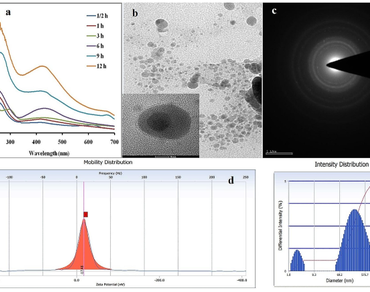
References
Abd Elhamid EM, Sadak MS, Tawfik MM (2016) Physiological response of fenugreek plant to the application of proline under different water regimes. Res J Pharm Biol Chem Sci 7(3):580–594
Akhtar MS, Khan AA, Swamy MK, Hakeem KR (2016) Impact of metal nanoparticles on the morphological and physiological changes in plants: a review. Nanotechnology 2(4):179–183. https://doi.org/10.15761/FNN.1000132
Akhtar MS, Panwar J, Yun YS (2013) Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain Chem Eng 1(6):591–602
Aravind R, Kumar A, Eapen SJ, Ramana KV (2009) Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett Appl Microbiol 48(1):58–64. https://doi.org/10.1111/j.1472-765x.2008.02486.x
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1–15. https://doi.org/10.1104/pp.24.1.1
Bakhoum GS, Sadak MS, Tawfik MM (2022) Chitosan and Chitosan nanoparticle effect on growth, productivity and some biochemical aspects of Lupinus termis L plant under drought conditions. Egypt J Chem 65(5):537–549. https://doi.org/10.21608/ejchem202197832.4563
Bakshi M, Liné C, Bedolla DE, Stein RJ, Kaegi R (2019) Assessing the impacts of sewage sludge amendment containing nano-TiO2 on tomato plants: a life cycle study. J Hazard Mater 369:191–198. https://doi.org/10.1016/j.jhazmat.2019.02.036
Bhanger MI, Bukhari SB, Memon S (2008) Antioxidative activity of extracts from a fenugreek seeds (Trigonella foenum-graecum). Pak J Anal Environ Chem 9(2):78–83
Boxall P, Purcell J, Wright PM (eds) (2008) Human resource management: scope, analysis and significance. The Oxford Handbook of Human Resource Management. Oxford University Press, Oxford, pp 1–18
Cvjetko P, Miloši´c A, Domijan A-M, Vinkovi´cVrˇcek I, Toli´c S, PeharecŠtefani´c P (2017) Toxicity of silver ions and differently coated silver nanoparticles in Alliumcepa roots. Ecotoxicol Environ Saf 137:18–28. https://doi.org/10.1016/j.ecoenv.2016.11.009
DeRosa MR, Monreal C, Schnitzer M, Walsh R, Sultan Y (2010) Nanotechnology in fertilizers. Nat Nanotechnol 5:91. https://doi.org/10.1038/nnano.2010.2
Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ (2013) Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol 47(2):1082–1090. https://doi.org/10.1021/es302973y
El-Bassiouny HMS, Abdallah MMS, El-Enany MAM, Sadak MS (2020) Nano-zinc oxide and arbuscular mycorrhiza effects on physiological and biochemical aspects of wheat cultivars under saline conditions. Pak J Biol Sci 23:478–490. https://doi.org/10.3923/pjbs.2020.478.490
Faiz S, Shah AA, Naveed NH, Nijabat A, Yasin NA, Batool AI, Ali A (2022) Synergistic application of silver nanoparticles and indole acetic acid alleviate cadmium induced stress and improve growth of Daucus carota L. Chemosphere 290:133200. https://doi.org/10.1016/j.chemosphere.2021.133200
Farghaly FA, Nafady NA (2015) Green synthesis of silver nanoparticles using leaf extract of Rosmarinus officinalis and its effect on Tomato and Wheat plants. J Agric Sci 7(11). https://doi.org/10.4103/pm.pm_226_17
Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13(4):400–408. https://doi.org/10.1038/nmat3890
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60(39):9781–9792. https://doi.org/10.1021/jf302154y
Gupta SD, Agarwal A, Pradhan S (2018) Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol Environ Saf 161:624–633. https://doi.org/10.1016/j.ecoenv.2018.06.023
Hojjat SS (2015) Impact of silver nanoparticles on germinated fenugreek seed. Int J Agric Crop Sci 8(4):627–630
Jain S, Mehata MS (2017) Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep 7(1): p.15867
Jasim B, Thomas R, Mathew J, Radhakrishnan EK (2017) Plant growth and diosgenin enhancement effect of silver nanoparticles in fenugreek (Trigonella foenum-graecum L). Saudi Pharm J 25(3):443–447. https://doi.org/10.1016/j.jsps.2016.09.012
Jhanzab HM, Razzaq A, Bibi Y, Yasmeen F, Yamaguchi H, Hitachi K, Tsuchida K, Komatsu S (2019) Proteomic analysis of the effect of inorganic and organic chemicals on silver nanoparticles in wheat. Int J Mol Sci 20(4):825. https://doi.org/10.3390/ijms20040825
Jiang HS, Qiu XN, Li GB, LiW, Yin LY (2014) Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ Toxicol Chem 33:1398–1405. https://doi.org/10.1002/etc.2577
Juárez-Maldonado A, Rosales-Velázquez JL, Ortega-Ortiz H, Cabrera-De-la-Fuente M, Ramírez H (2013) Accumulation of silver nanoparticles and its effect on the antioxidant capacity in Allium cepa L. Phyton 82(1):91–97. https://doi.org/10.32604/PHYTON.2013.82.091
Kaveh R, Li YS, Ranjbar S, Tehrani R, Brueck CL, Van Aken B (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47:10637–10644. https://doi.org/10.1021/es402209w
Khan S, Zahoor M, Khan RS, Ikram M, Islam NU (2023) The impact of silver nanoparticles on the growth of plants: the agriculture applications. Heliyon. https://doi.org/10.1016/j.heliyon.2023.e16928
Latif HH, Ghareib M, Tahon MA (2017) Phytosynthesis of silver nanoparticles using leaf extracts from Ocimum basilicum and Mangifira indica and their effect on some biochemical attributes of Triticum aestivum. Gesunde Pflanzen 69(1):39–46
Lee WM, Kwak JI, An YJ (2012) Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: media effect on phytotoxicity. Chemosphere 86(5):491–499. https://doi.org/10.1016/j.chemosphere.2011.10.013
Madar Z, Abel R, Samish S, Arad J (1988) Glucose-lowering effect of fenugreek in non-insulin dependent diabetics. Eur J Clin Nutr 42(1):51–54
Majumder DD, Ulrichs C, Majumder D, Mewis I, Thakur AR (2007) Current status and future trends of nanoscale technology and its impact on modern computing, biology, medicine and agricultural biotechnology. In: International Conference on Computing: Theory and Applications (ICCTA’07) (563–573). IEEE
Malik WA, Mahmood I, Razzaq A, Afzal M, Shah GA, Iqbal A, Ye W (2021) Exploring potential of copper and silver nano particles to establish efficient callogenesis and regeneration system for wheat (Triticum aestivum L). GM Crops food 12(1):564–585. https://doi.org/10.1080/21645698.2021.1917975
Monica RC, Cremonini R (2009) Nanoparticles and higher plants. Caryologia 62(2):161–165. https://doi.org/10.1080/00087114.2004.10589681
Moradikor N, Bayati Zadeh J, Moradikor J (2013) Physiological and pharmaceutical effects of Tribulus terrestris as a multipurpose and valuable medicinal plant. Int J Adv Biol Biomed Res 1(5):556–562
Mughal SS, Hassan SM (2022) Comparative study of AgO nanoparticles synthesize via biological, chemical and physical methods: a review. Am J Mater Synth Proc 7(2):15–28
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179(3):154–163. https://doi.org/10.1016/j.plantsci.2010.04.012
Najafi S, Jamei R (2014) Effect of silver nanoparticles and pb(NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J Stress Physiol Biochem 10:316–325
Ndlovu N, Mayaya T, Muitire C, Munyengwa N (2020) Nanotechnology applications in crop production and food systems. Int J Plant Breed
Pallavi, Mehta CM, Srivastava R, Arora S, Sharma AK (2016) Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. Biotech 6:254. https://doi.org/10.1007/s13205-016-0567-7
Parthasarathy VA (2008) Organic spices. New India Publishing
Qian H, Peng X, Han X, Ren J, Sun L, Fu Z (2013) Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J Environ Sci 25(9):1947–1956. https://doi.org/10.1016/S1001-0742(12)60301-5
Racuciu M, Creanga DE (2007) TMA-OH Coated magnetic nanoparticles internalized in vegetal tissue. Roman J Phys 52:395–402
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Razzaq A, Ammara R, Jhanzab HM, Mahmood T, Hafeez A, Hussain S (2016) A novel nanomaterial to enhance growth and yield of wheat. J Nanosci Technol 2(1):55–58
Sadak MS (2019) Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull Natl Res Cent 43(1):1–6
Sadak MS (2016) Mitigation of drought stress on fenugreek plant by foliar application of trehalose. Inter J Chem Tech Res 9(2):147–155
Sadak MS, Tawfik MM, Bakhoum GS (2022) Role of chitosan and chitosan-based nanoparticles on drought tolerance in plants: probabilities and prospects. In: Nanomaterial-Plant Interactions Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences, Edited by: Kumar S and Madihally SV. https://doi.org/10.1016/B978-0-323-85391-0.00013-7
Saeideh N, Rashid J (2014) Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J Stress Physiol Biochem 10(n.1)
Salachna P, Byczyńska A, Zawadzińska A, Piechocki R, Mizielińska M (2019) Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 9(10):610. https://doi.org/10.3390/agronomy9100610
Salama HM (2012) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L). Int Res J Biotechnol 3(10):190–197
Sharma P, Bhatt D, Zaidi MGH, Saradhi PP, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167(8):2225–2233. https://doi.org/10.1007/s12010-012-9759-8
Sharon M, Choudhary AK, Kumar R (2010) Nanotechnology in agricultural diseases and food safety. J Phytol 2:78–82
Sheykhbaglou R, Sedghi M, Shishevan MT, Sharifi RS (2010) Effects of nano-iron oxide particles on agronomic traits of soybean. Notulae Scientia Biologicae 2(2):112–113. https://doi.org/10.15835/NSB224667
Siddiqi KS, Husen A (2022) Plant response to silver nanoparticles: a critical review. Crit Rev Biotechnol 42(7):973–990. https://doi.org/10.1080/07388551.2021.1975091
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill). Saudi J Biol Sci 21(1):13–17. https://doi.org/10.1016/j.sjbs.2013.04.005
Siddiqui MH, Al-Whaibi MH, Firoz M (2015) Nanotechnology and Plant sciences. Springer Int Publishing Switz 19–35
Singh G, Babele PK, Shahi SK, Sinha RP, Tyagi MB, Kumar A (2014a) Green synthesis of silver nanoparticles using cell extracts of Anabaena doliolum and screening of its antibacterial and antitumor activity. J Microbiol Biotechnol 24(10):1354–1367. https://doi.org/10.4014/jmb.1405.05003
Singh G, Babele PK, Shahi SK, Sinha RP, Tyagi MB, Kumar A (2014b) Green synthesis of silver nanoparticles using cell extracts of Anabaena doliolum and screening of its antibacterial and antitumor activity. J Microbiol Biotechnol 24(10):1354–1367
Syu YY, Hung JH, Chen JC, Chuang HW (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64. https://doi.org/10.1016/j.plaphy.2014.07.010
Tripathi DK, Singh S, Singh S, Srivastava PK, Singh VP (2017) Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem 110:167–177. https://doi.org/10.1016/j.plaphy.2016.06.015
Vannini C, Domingo G, Onelli E, Prinsi B, Marsoni M (2013) Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE 8(7):68752. https://doi.org/10.1371/journal.pone.0068752
Vinković T, Novák O, Strnad M, Goessler W, Jurašin DD (2017) Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ Res 156:10–18. https://doi.org/10.1016/j.envres.2017.03.015
Wijnhoven SW, Peijnenburg WJ, Herberts CA, Hagens WI, Oomen AG (2009) Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3(2):109–138. https://doi.org/10.1080/17435390902725914
Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES (2012) Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 7:e47674. https://doi.org/10.1371/journal.pone.0047674
Author Information
Department of Botany, Gargi College, University of Delhi, Delhi, India