Effect of heat stress on wild type and A7a knockout mutant Arabidopsis thaliana plants
Patel Kajal, Bidalia Ankita, Tripathi Indu, Gupta Yamal, Arora Priyanka, Rao K. S.
Research Articles | Published: 30 July, 2021
First Page: 168
Last Page: 178
Views: 4820
Keywords: Abiotic stress, Knockout mutant, Plant performance, Thermo-tolerance
Abstract
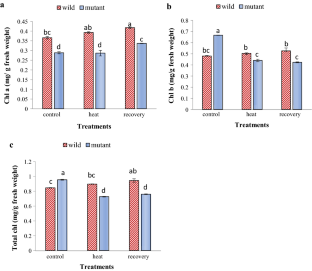
Rise in temperature causes heat stress which is a major global risk that limits plant growth, metabolism and productivity. Plants possess various strategies and have numerous mechanisms at various levels like morphological, biochemical, physiological levels to withstand high temperature conditions. At molecular level, in Arabidopsis thaliana class A HSFs specifically AtHsfA1b, AtHsfA1d, AtHsfA7a act as activators of transcription reported to have a positive feedback during heat stress which helps in thermo-tolerance. Hence, this study was carried out to understand the role of transcription factor HsfA7a gene for the ability of sustaining heat stress by A. thaliana. Two ecotypes of A. thaliana, Col-0 (wild Columbia type) and HsfA7a knockout mutant were used for the study. Various morphological, biochemical and physiological parameters were analysed to evaluate the performance of the plants under stress. For treatment, 38 °C temperature (heat stress) for 24 h followed by a recovery of 24 h was used which were compared with plants grown under normal conditions. Consequently, it was found that heat stress and recovery both had significant effects on both the ecotypes whereas wild type was found to perform better under heat stress compared to the mutant. Thus, it can be concluded that HsfA7a gene is playing a key role in thermo-tolerance in A. thaliana, similar to other class A HSFs.
References
Ainsworth AE, Gillespie MK (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Alaei Y (2011) The effect of amino acids on leaf chlorophyll content in bread wheat genotypes under drought stress conditions. Middle East J Sci Res 10:99–101
Arnon ID (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Arora P, Bidalia A, Rao KS (2016) Growth and photosynthetic response of wheat and mustard plants to intercropping. Phytomorphology 66:35–44
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Batool A, Wahid A, Abbas G, Shah SH, Naeem M, Akhtar NP, Hassnain Z (2019) Application of Moringa oleifera plant extracts for enhancing the concentration of photosynthetic pigments leading to stable photosynthesis under heat stress in maize (Zea mays L.). Pak J Bot 51:3021–3026. https://doi.org/10.30848/pjb2019-6(20)
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. https://doi.org/10.1016/s0023-6438(95)80008-5
Busch W, Wunderlich M, Schöffl F (2005) Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J 41:1–14. https://doi.org/10.1111/j.1365-313x.2004.02272.x
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11:163–177. https://doi.org/10.1186/1471-2229-11-163
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37:201–204. http://nopr.niscair.res.in/handle/123456789/15379
Duveiller E, Singh RP, Nicol JM (2007) The challenges of maintaining wheat productivity: pests, diseases and potential epidemics. Euphytica 157:417–430. https://doi.org/10.1007/s10681-007-9380-z
Friedrich T, Oberkofler V, Trindade I, Altmann S, Brzezinka K, Lämke J, Gorka M, Kappel C, Sokolowska E, Skirycz A, Graf A (2021) Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat Commun 12:1–15. https://doi.org/10.1038/s41467-021-23786-6
Gaspar T, Franck T, Bisbis B, Kevers C, Jouve L, Hausman JF, Dommes J (2002) Concepts in plant stress physiology: Application to plant tissue cultures. Plant Growth Regul 37:263–285. https://doi.org/10.1023/a:1020835304842
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553. https://doi.org/10.1046/j.1365-3040.1998.00309.x
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 4:9643–9684. https://doi.org/10.3390/ijms14059643
Heckwolf M, Pater D, Hanson DT, Kaldenhoff R (2011) The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J 67:795–804. https://doi.org/10.1111/j.1365-313x.2011.04634.x
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. https://doi.org/10.1007/s004250050524
Jin B, Wang L, Wang J, Jiang KZ, Wang Y, Jiang XX, Ni CY, Wang YL, Teng NJ (2011) The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana. BMC Plant Biol 11:1–10. https://doi.org/10.1186/1471-2229-11-35
Khayatnezhad M, Gholamin R, Jamaati-e-Somarin S, Zabihi-e-Mahmoodabad R (2011) The leaf chlorophyll content and stress resistance relationship considering in corn cultivars (Zea mays). Adv Environ Biol 5:118–122. https://doi.org/10.5897/ajmr11.964
Kipp E, Boyle M (2013) The effects of heat stress on reactive oxygen species production and chlorophyll concentration in Arabidopsis thaliana. Res Plant Sci 1:20–23. https://doi.org/10.12691/plant-1-2-3
Kishor PBK, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438. http://www.jstor.org/stable/24110209
Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146:748–761. https://doi.org/10.1104/pp.107.112060
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682. https://doi.org/10.1016/j.plaphy.2010.05.005
Li XD, Wang XL, Cai YM, Wu JH, Mo BT, Yu ER (2017) Arabidopsis heat stress transcription factors A2 (HSFA2) and A3 (HSFA3) function in the same heat regulation pathway. Acta Physiol Plant 39:67. https://doi.org/10.1007/s11738-017-2351-7
Lichtenthaler HK (1987) Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J Plant Physiol 131:101–110. https://doi.org/10.1016/S0176-1617(87)80271-7
Lin KF, Tsai MY, Lu CA, Wu SJ, Yeh CH (2018) The roles of Arabidopsis HSFA2, HSFA4a, and HSFA7a in the heat shock response and cytosolic protein response. Bot Stud 59:15–24. https://doi.org/10.1186/s40529-018-0231-0
Liu HC, Charng YY (2013) Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol 163:276–290. https://doi.org/10.1104/pp.113.221168
Liu HC, Liao HT, Charng YY (2011) The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ 34:738–751. https://doi.org/10.1111/j.1365-3040.2011.02278.x
Lohmann C, Eggers-Schumacher G, Wunderlich M, Schöffl F (2004) Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol Genet Genom 271:11–21. https://doi.org/10.1007/s00438-004-1001-0
Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD (2002) In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev 16:1555–1567. https://doi.org/10.1101/gad.228802
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van BF (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309. https://doi.org/10.1016/j.tplants.2011.03.007
Nover N, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf KD (2001) Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperones 6:177–189. https://doi.org/10.1379/1466-1268(2001)006%3C0177:aathst%3E2.0.co;2
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882. https://doi.org/10.1093/jxb/erq340
Saeidian S (2014) Activity of Guaiacol Peroxidase of Solanum lycopersicum in presence of detergents and chaotropic Agents. IOSR J Pharm 4:01–05. https://doi.org/10.9790/3013-04020101-05
Scharf KD, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. BBA Gene Regul Mech 1819:104–119. https://doi.org/10.1016/j.bbagrm.2011.10.002
Shahriari R (1999) Of cold tolerance in wheat (Doctoral dissertation, M. Sc. Thesis Plant Breeding. Islamic Azad University of Ardabil)
Sivasubramanian R, Mukhi N, Kaur J (2015) Arabidopsis thaliana: a model for plant research. In: Plant biology and biotechnology (pp 1–26). Springer, New Delhi. https://doi.org/10.1007/978-81-322-2283-5_1
Skirycz A, Claeys H, De Bodt S, Oikawa A, Shinoda S, Andriankaja M, Maleux K, Eloy NB, Coppens F, Yoo SD, Saito K (2011) Pause-and-stop: the effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23:1876–1888. https://doi.org/10.1105/tpc.111.084160
Sung DY, Guy CL (2003) Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis: evidence for pleiotropic consequences. Plant Physiol 132:979–987. https://doi.org/10.1104/pp.102.019398
The Intergovernmental Panel on Climate Change (IPCC) (2018) Special report: Global warming of 1.5 °C (IPCC, Incheon,Korea, 2018). www.ipcc.ch/report/sr15/
Todorova D, Katerova Z, Shopova E, Jodinskienė M, Jurkonienė S, Sergiev I (2016) Responses of pea plants to heat stress and spermine treatment Karščio ir apdorojimo sperminu įtaka žirniams. Žemdirbystė 103:99–106. https://doi.org/10.13080/z-a.2016.103.013
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252. https://doi.org/10.1016/j.tplants.2004.03.006
Xu Q, Henry RL, Guikema JA, Paulsen GM (1995) Association of high-temperature injury with increased sensitivity of photosynthesis to abscisic acid in wheat. Environ Exp Bot 35:441–449. https://doi.org/10.1016/0098-8472(95)00040-2
Yin H, Chen Q, Yi M (2008) Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul 54:45–54. https://doi.org/10.1007/s10725-007-9227-6
Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim J-M, Seki M, Todaka D, Osakabe Y, Sakuma Y, Schoffl F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol Genet Genom 286:321–332. https://doi.org/10.1007/s00438-011-0647-7
Author Information
Department of Botany, University of Delhi, New Delhi, India