Effect of zinc deficiency and excess on catalase activity and HvCAT2 gene expression in barley
Batova Yuliya, Kaznina Natalia, Repkina Natalia, Titov Alexander
Short Communications | Published: 11 April, 2022
First Page: 833
Last Page: 838
Views: 3813
Keywords: Hordeum vulgare (L), Zinc, Antioxidant enzymes, Lipid peroxidation, Oxidative stress
Abstract
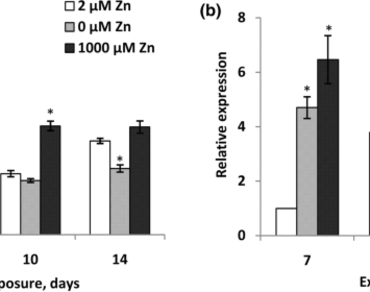
The zinc (Zn) is an important microelement for all living organisms but its deficiency or excess caused negative effect on metabolism. The aim of this study was to investigate the effect of Zn deficiency and excess on total catalase (CAT) activity and HvCAT2 gene expression, encoding one of the enzyme isoforms in barley leaves. It was shown, that deficiency and excess of Zn resulted in decrease in growth and biomass accumulation of barley seedlings. The Zn deficiency (0 µM) did not affect the MDA content in leaves, in contrast the Zn excess (1000 µM) caused increase in MDA level that is marker reaction of developing oxidative stress. Both Zn deficiency and excess resulted in significant increase in HvCAT2 transcripts accumulation in leaves of barley, on the day 7 of experiment. Under the Zn deficiency the mRNA of HvCAT2 decreased on day 14 while the CAT activity did not change which indicates to the normalization of redox balance of cells. However, Zn excess resulted in decline in CAT activity despite the high HvCAT2 transcripts amount and developing oxidative stress that can be an evidence of involvement of supplementary mechanisms of protection and/or reparation. In general, the results showed the participation of CAT and the HvCAT2 gene in the adaptation of barley plants to both Zn deficiency and an excess.
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Alam NB, Ghosh A (2018) Comprehensive analysis and transcript profiling of Arabidopsis thaliana and Oryza sativa catalase gene family suggest their specific role in development and stress responses. Plant Physiol Biochem 123:54–64. https://doi.org/10.1016/j.plaphy.2017.11.018
Alloway BJ (2008) Zinc in soils and crop nutrition. IZA, Brussels
Anjum NA, Singh HP, Khan MIR, Masood A, Per TS, Negi A, Batish DR, Khan NA, Duarte AC, Pereira E, Ahmad I (2015) Too much is bad—an appraisal of phytotoxicity of elevated plant-beneficial heavy metal ions. Environ Sci Pollut Res 22:3361–3382. https://doi.org/10.1007/s11356-014-3849-9
Auh C-K, Scandalios JG (1997) Spatial and temporal responses of the maize catalases to low temperature. Physiol Plant 101:149–156
Blasco B, Graham NS, Broadley MR (2015) Antioxidant response and carboxylate metabolism in Brassica rapa exposed to different external Zn, Ca and Mg supply. J Plant Physiol 176:16–24. https://doi.org/10.1016/j.jplph.2014.07.029
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Broadley MR (2007) Zinc in plants. New Phytol 173:677–702. https://doi.org/10.1111/j.1469-8137.2007.01996.x
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205. https://doi.org/10.1046/j.1469-8137.2000.00630.x
Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radical Biol Med 122:4–20. https://doi.org/10.1016/j.freeradbiomed.2018.01.011
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Van Breusegem IDF (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795. https://doi.org/10.1007/s000180050041
Du YY, Wang PC, Chen J, Song CP (2008) Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol 50:1318–1326. https://doi.org/10.1111/j.1744-7909.2008.00741.x
Hajiboland R, Amirazad F (2010) Growth, photosynthesis and antioxidant defense system in Zn-deficient red cabbage plants. Plant Soil Environ 56:209–217. https://doi.org/10.17221/207/2009-PSE
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Kabata-Pendias A (2010) Trace elements in soils and plants. Taylor and Fransis Group, Boca Raton. https://doi.org/10.1017/S0014479711000743
Karpets YV, Kolupaev YE, Yastreb TO, Oboznyi AI (2015) Effects of NO-status modification, heat hardening, and hydrogen peroxide on the activity of antioxidant enzymes in wheat seedlings. Russ J Plant Physiol 62:292–298. https://doi.org/10.1134/S1021443715030097
Leung DWM (2018) Studies of catalase in plants under abiotic stress. In: Gupta DK et al (eds) Antioxidants and antioxidant enzymes in higher plants. Springer International Publishing AG, Berlin, pp 27–39. https://doi.org/10.1007/978-3-319-75088-0
Li X, Yang Y, Jia L, Chen H, Wei X (2013) Zn-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol Environ Saf 89:150–157. https://doi.org/10.1016/j.ecoenv.2012.11.025
Lin Y-F, Aarts MGM (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69:3187–3206. https://doi.org/10.1007/s00018-012-1089-z
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 –ΔΔCt method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Maret W (2019) The redox biology of redox-inert zinc ions. Free Radic Biol Med 134:311–326. https://doi.org/10.1016/j.freeradbiomed.2019.01.006
Mattiello EM, Ruiz HA, Neves JCL, Ventrella MC (2015) Zinc deficiency affects physiological and anatomical characteristics in maize leaves. J Plant Physiol 183:138–143. https://doi.org/10.1016/j.jplph.2015.05.014
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220. https://doi.org/10.1093/jxb/erq282
Mhamdi A, Noctor G, Baker A (2012) Plant catalases: peroxisomal redox guardians. Arch Biochem Biophys 525:181–194. https://doi.org/10.1016/j.abb.2012.04.015
Mukhopadhyoy M, Das A, Subba P, Bantawa P, Sarkar B, Ghosh P, Mondal TK (2013) Structural, physiological, and biochemical profiling of tea plants under zinc stress. Biol Plant 57:474–480. https://doi.org/10.1007/s10535-012-0300-2
Radyuk MS, Domanskaya IN, Shcherbakov RA, Shalygo NV (2009) Effect of low above-zero temperature on the content of low-molecular antioxidants and activities of antioxidant enzymes in green barley leaves. Russ J Plant Physiol 56:175–180. https://doi.org/10.1134/S1021443709020058
Raza A, Su W, Gao A, Mehmood SS, Hussain MA, Nie W, Lv Y, Zou X, Zhang X (2021) Catalase (CAT) gene family in rapeseed (Brassica napus L.): genome-wide analysis, identification, and expression pattern in response to multiple hormones and abiotic stress conditions. Int J Mol Sci 22:4281. https://doi.org/10.3390/ijms22084281
Rehman A, Farooq M, Ozturk L, Asif M, Siddique KHM (2018) Zinc nutrition in wheat-based cropping systems. Plant Soil 422:283–315. https://doi.org/10.1007/s11104-017-3507-3
Sharma PN, Kumar P, Tewari RK (2004) Early signs of oxidative stress in wheat plants subjected to zinc deficiency. J Plant Nutr 27:451–463. https://doi.org/10.1081/PLN-120028873
Sinclair SA, Krämer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta 1823:1553–1567. https://doi.org/10.1016/j.bbamcr.2012.05.016
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Skadsen RW, Schulze-Lefert P, Herbst JM (1995) Molecular cloning, characterization and expression analysis of two catalase isozyme genes in barley. Plant Mol Biol 29:1005–1014
Smeets K, Ruytinx J, Semane B, Van Belleghem F, Remans T, Van Sanden S, Vangronsveld J, Cuypers A (2008) Cadmium-induced transcriptional and enzymatic alterations related to oxidative stress. Environ Exp Bot 63:1–8. https://doi.org/10.1016/j.envexpbot.2007.10.028
Tewari RK, Kumar P, Sharma PN (2008) Morphology and physiology of zinc-stressed mulberry plants. J Plant Nutr Soil Sci 171:286–294. https://doi.org/10.1002/jpln.200700222
Tewari RK, Kumar P, Sharma PN (2019) An effective antioxidant defense provides protection against zinc deficiency-induced oxidative stress in Zn-efficient maize plants. J Plant Nutr Soil Sci 182:701–707. https://doi.org/10.1002/jpln.201800622
Tounsi S, Kamoun Y, Feki K, Jemli S, Saïdi MN, Ziadi H, Alcon C, Brini F (2019) Localization and expression analysis of a novel catalase from Triticum monococcum TmCAT1 involved in response to different environmental stresses. Plant Physiol Biochem 139:366–378. https://doi.org/10.1016/j.plaphy.2019.03.039
Tyagi S, Shumayla M, Singh K, Upadhyay SK (2021) Molecular characterization revealed the role of catalases under abiotic and arsenic stress in bread wheat (Triticum aestivum L.). J Hazar Mater 403:123585. https://doi.org/10.1016/j.jhazmat.2020.123585
Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75:1468–1476. https://doi.org/10.1016/j.chemosphere.2009.02.033
Willekens H, Inzé D, Van Montagu M, van Camp W (1995) Catalases in plants. Mol Breed 1:207–228
Author Information
Institute of Biology of the Karelian Research Centre of the Russian Academy of Sciences, Petrozavodsk, Russia