Effects of seed priming on germination and abiotic stress responses in Gymnocladus assamicus ex P.C. Kanjilal
*Article not assigned to an issue yet
Research Articles | Published: 04 December, 2025
First Page: 0
Last Page: 0
Views: 78
Keywords: n Gymnocladus assamicusn , Seed priming, Germination behavior, Morpho-physiological responses, Critically endangered Himalayan plant
Abstract
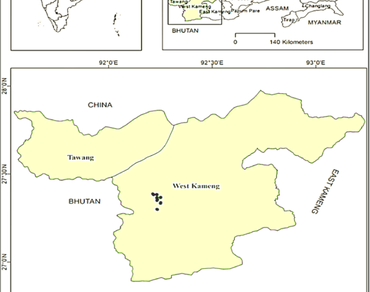
The critically endangered tree species Gymnocladus assamicus ex P.C. Kanjilal, endemic to the Eastern Himalayas, faces severe challenges to its regeneration and survival due to habitat loss and changing climatic conditions. To explore strategies that enhance its propagation and stress resilience, two independent experiments were conducted. In the first experiment, the effects of different seed priming treatments were evaluated on germination behavior. Seed priming, particularly water soaking combined with heat treatment or GA3 (50 ppm), significantly improved germination and physiological performance under both control and stress conditions. Seed priming significantly improved germination percentage, rate, and uniformity compared to unprimed controls, indicating its effectiveness in overcoming dormancy and promoting early seedling establishment. In the second experiment, unprimed seedlings were exposed to various abiotic stresses, i.e., heat, cold, drought, submergence, and low pH to assess their morphological and physiological responses. Stress treatments markedly reduced growth parameters, membrane stability, and chlorophyll fluorescence efficiency (Fv/Fm), while increasing relative electrolyte leakage. Histochemical staining using Schiff’s reagent, Evans Blue, Nitroblue Tetrazolium (NBT), and 3,3′-Diaminobenzidine (DAB) confirmed oxidative stress and associated cellular damage. Together, these findings highlight the potential of seed priming as an effective pre-sowing strategy to enhance germination and early establishment, while also providing insights into the abiotic stress tolerance mechanisms of G. assamicus. Such integrated understanding contributes to the development of informed conservation and restoration strategies for this endangered Himalayan species.
References
Aslam M, Fakher B, Ashraf MA, Cheng Y, Wang B, Qin Y (2022) Plant low-temperature stress: signaling and response. Agron 12(3):702. https://doi.org/10.3390/agronomy12030702
Awasthi JP, Saha B, Regon P, Sahoo S, Chowra U, Pradhan A, Roy A, Panda SK (2017) Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS ONE 12(4):e0176357. https://doi.org/10.1371/journal.pone.0176357
Baruah PM, Agarwala N, Bordoloi KS, Regon P, Tanti B (2025) Long non-coding RNAs responsive to temperature stress conditions in tea plants. J Plant Growth Regul 44(4):1728–1752. https://doi.org/10.1134/S106235902560028X
Bascuñán-Godoy L, Uribe E, Sanhueza D, Carvajal DE, Franck N (2018) Drought and heat stress effects on photosynthesis in Pinus sylvestris. Tree Physiol 38(8):1230–1241. https://doi.org/10.1093/treephys/tpy049
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, Cambridge
Chen K, Arora R (2013) Priming memory invokes seed stress-tolerance. Environ Exp Bot 94:33–45. https://doi.org/10.1016/j.envexpbot.2012.03.005
Choudhury BI, Khan ML, Arunachalam A, Arunachalam K (2007a) Gymnocladus assamicus Kanjilal ex P.C. Kanjilal fruit - a soap substitute. Nat Prod Radiance 6:427–429
Choudhury BI, Khan ML, Arunachalam A, Das AK (2007b) Ecology and conservation of the critically endangered tree species Gymnocladus assamicus in Arunachal Pradesh. India Int J Ecol 1:059282. https://doi.org/10.1155/2007/59282
Daudi A, O’brien JA (2012) Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-Protoc 2(18):e263
Farooq M, Gogoi N, Barthakur S, Baroowa B, Bharadwaj N, Alghamdi SS, Siddique KHM (2019) Drought stress in grain legumes during reproduction and grain filling. J Agron Crop Sci 205(4):249–264. https://doi.org/10.1111/jac.12318
Ganeshaiah KN (2005) Recovery of endangered and threatened species: developing a national priority list of plants and insects. Curr Sci 89:599–600
Gupta S, Mao AA, Sarma S, Satish T (2017) Responses of pretreatment and nutrient media on seed germination in Gymnocladus assamicus, a critically endangered legume tree species from North-East India. J Tree Sci 36(1):86–92. https://doi.org/10.5958/2455-7129.2017.00012.7
Hussain S, Hussain S, Qadir T, Anjum SA (2020) Seed priming strategies in field crops—a review. Plant Growth Regul 92:23–45. https://doi.org/10.1007/s10725-020-00624-y
Islam MA, Khan A, Hoque MA (2016) Effect of seed priming on germination and seedling growth of Acacia auriculiformis. J Forestry Res 27(4):797–805. https://doi.org/10.1007/s11676-016-0212-1
Jyoti SY, Tanti B (2024) Low pH and water stress induced morpho-physiological response in some traditional Citrus species of Assam. India Biol Bull 51(3):625–643. https://doi.org/10.1134/S1062359023604111
Jyoti SY, Kalita I, Tanti B (2024) Phytochemical screening, proximate composition and antioxidant activities of Citrus germplasm of Assam. India Vegetos 37(3):1153–1165. https://doi.org/10.1007/s42535-023-00666-6
Kalita I, Tanti B (2025) Impact of abiotic stress on biochemical profiles and antioxidant defense mechanisms in Gymnocladus assamicus ex. P.C. Kanjilal: insights into the survival strategies of a critically endangered Himalayan Plant. Biol Bull 52:203. https://doi.org/10.1134/S106235902560028X
Kalita B, Sarma K, Bawri A, Tanti B (2024) Assessing population density and regeneration potential of Paris polyphylla Sm. in the Arunachal Himalaya India: implications for conservation. Vegetos. https://doi.org/10.1007/s42535-024-01087-9
Kapoor N, Pande V (2015) Effect of salt stress on growth parameters, moisture content, relative water content and photosynthetic pigments of fenugreek variety RMt-1. J Plant Sci 10(6):210–221. https://doi.org/10.3923/jps.2015.210.221
Khan MA, Zafar M, Ullah S (2021) Physiological and biochemical responses of Eucalyptus globulus under drought stress. Trees 35(6):1769–1782. https://doi.org/10.1007/s00468-021-02149-4
Kundu BK, Regon P, Phukan SG, Borgohain P, Agarwala N, Tanti B (2025) Responses of aromatic Keteki Joha rice to varying nitrogen levels: impacts on grain yield, antioxidant defense mechanisms, and stress resilience. Plant Sci 359:112626. https://doi.org/10.1016/j.plantsci.2025.112626
Lamaoui M, Jemo M, Datla R, Bekkaoui F (2018) Heat and drought stresses in crops and approaches for their mitigation. Front Chem 6:26. https://doi.org/10.3389/fchem.2018.00026
Li L, Wu D, Zhen Q, Zhang J, Qiu L, Huang G, Yang C (2021) Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae). Open Life Sci 16(1):455–463. https://doi.org/10.1515/biol-2021-0049
Lukin SV, Zavalin AA, Sokolov OA, Shmyreva NY (2019) Legume reaction to soil acidity. Amazon Investig. 8(23):162–70. https://www.amazoniainvestiga.info/index.php/amazonia/article/view/800
Menon S, Choudhury BI, Khan ML, Peterson AT (2010) Ecological niche modeling and local knowledge predict new populations of Gymnocladus assamicus a critically endangered tree species. Endanger Species Res 11(2):175–181. https://doi.org/10.3354/esr00275
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33(4):453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mitra PP, Loqué D (2014) Histochemical staining of Arabidopsis thaliana secondary cell wall elements. Journal of Visualized Experiments: Jove 13(87):51381. https://doi.org/10.3791/51381
Mor V, Dalal PK, Sharma S, Bhuker A, PR MS (2024) Seed priming to enhance plant stand under stressed environment: a review. Seed Res 49(1):59–71. https://doi.org/10.56093/sr.v49i1.153450
Nadeem SM, Imran M, Naveed M, Khan MY, Ahmad M, Zahir ZA, Crowley DE (2017) Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J Sci Food Agric 97(15):5139–5145. https://doi.org/10.1002/jsfa.8393
Qureshi SN, Wani MS, Javaid K (2016) Effect of stratification and gibberellic acid on seed germination and seedling growth of walnut under Kashmir valley conditions. Indian J Hortic 73(02):171–176. https://doi.org/10.5958/0974-0112.2016.00042.6
Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D (2012) Seed germination and vigor. Annu Rev Plant Biol 63:507–533. https://doi.org/10.1146/annurev-arplant-042811-105550
Rao RR, Hajra PK (1986) Floristic diversity of the eastern Himalaya—in a conservation perspective. In: Proceedings. Animal sciences, IASc pp 131–151
Ray D, Saha S, Chowdhury A (2023) Physiological adaptations of Dalbergia sissoo to drought and heat stress. Plant Stress 8:100146. https://doi.org/10.1016/j.stress.2022.100146
Regon P, Dey S, Chowardhara B, Saha B, Kar S, Tanti B, Panda SK (2021) Physio-biochemical and molecular assessment of iron (Fe2+) toxicity responses in contrasting indigenous aromatic Joha rice cultivars of Assam, India. Protoplasma 258(2):289–299. https://doi.org/10.1007/s00709-020-01574-1
Regon P, Dey S, Rehman M, Pradhan AK, Chowra U, Tanti B, Talukdar AD, Panda SK (2022) Transcriptomic analysis revealed reactive oxygen species scavenging mechanisms associated with ferrous iron toxicity in aromatic Keteki Joha rice. Front Plant Sci 13:798580. https://doi.org/10.3389/fpls.2022.798580
Regon P, Saha B, Jyoti SY, Gupta D, Kundu B, Tanti B, Panda SK (2024) Transcriptional networks revealed late embryogenesis abundant genes regulating drought mitigation in aromatic Keteki Joha rice. Physiol Plant 176(3):e14348. https://doi.org/10.1111/ppl.14348
Saikia D, Tanti B (2024) Assessment of defense-associated antioxidant mechanism in banana under different abiotic stresses. Vegetos 37(4):1346–1356. https://doi.org/10.1007/s42535-023-00685-3
Sairam RK, Deshmukh PS, Shukla DS (2000) Tolerance of drought and temperature stress in relation to antioxidant enzyme activity in wheat genotypes. J Agron Crop Sci 184(1):55–61. https://doi.org/10.1046/j.1439-037x.2000.00363.x
Sarma K, Roy SJ, Kalita B, Regon P, Bawri A, Borah S, Nath MJ, Baruah UD, Sahariah D, Saikia A, Tanti B (2024) Distribution mapping of five threatened medicinally important plant species of Arunachal Himalaya. Vegetos 37(3):844–858. https://doi.org/10.1007/s42535-023-00619-z
Author Information
Department of Botany, Gauhati University, Guwahati, India