Enhanced seed germination of three Aristolochia species using light, karrikinolide and GA3
Research Articles | Published: 01 June, 2023
First Page: 1051
Last Page: 1060
Views: 4358
Keywords: n Aristolochian , Gibberellin, Light, Karrikinolide, Seed germination
Abstract
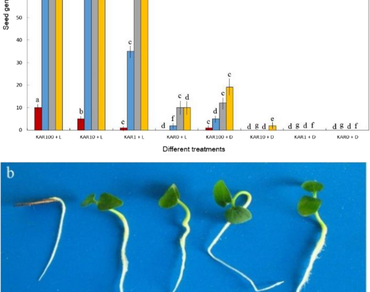
Aristolochia species form a large taxon and are used as traditional medicines. There are very few reports on seed germination of Aristolochia species. In this study, the effects of light and karrikinolide (KAR1) (1–100 nM) on seed germination of three sympatric Aristolochia species (A. labiata, A. debilis and A. ringens) from South China were investigated. The shortest germination period of the three species treated with both light and KAR1 was 8, 12 and 18 d, respectively. The percentage of seed germination in light was higher than in the dark in all three species, indicating that the seeds were light-sensitive and physiologically dormant. In the dark, the seeds of A. labiata and A. ringens could not germinate while only some A. debilis seeds germinated. After treatment with KAR1, A. debilis and A. labiata seeds germinated at a low frequency in the dark. However, A. ringens seeds could not germinate, even when exposed to a high concentration of KAR1. To investigate a possible functional mechanism of KAR1, A. labiata seeds were treated with 100 µM of some plant growth regulators, indole-3-acetic acid (IAA), abscisic acid (ABA), kinetin (KIN), gibberellic acid (GA3) and KAR1 (100 nM), and placed in the dark. Although the KAR1 + GA3 combination stimulated some seed germination, the seed germination in response to KAR1 and GA3 was not as efficient as in light. KIN, ABA, and IAA did not induced any seed germination in the dark.
References
Adams CA, Baskin JM, Baskin CC (2005a) Comparative morphology of seeds of four closely related species of Aristolochia subgenus Siphisia (Aristolochiaceae, Piperales). Bot J Linn Soc 148:433–436. https://doi.org/10.1111/j.1095-8339.2005.00402.x
Adams CA, Baskin JM, Baskin CC (2005b) Trait stasis versus adaptation in disjunct relict species: evolutionary changes in seed dormancy-breaking and germination requirements in a subclade of Aristolochia subgenus Siphisia (Piperales). Seed Sci Res 15:161–173. https://doi.org/10.1079/SSR2005207
Akindele AJ, Wani Z, Mahajan G, Sharma S, Aigbe FR, Naresh Satti N, Adeyemi OO, Mondhe DM (2015) Anticancer activity of Aristolochia ringens vahl. J Tradit Complement Med 5:35–41. https://doi.org/10.1016/j.jtcme.2014.05.001. Aristolochiaceae
Alves Silva da D, Borghetti F, Thompson K, Pritchard H, Grime JP (2011) Underdeveloped embryos and germination in Aristolochia galeata seeds. Plant Biol 13:104–108. https://doi.org/10.1111/j.1438-8677.2009.00302.x
Baskin CC, Baskin JM (1998) Seeds: Ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego, USA, p 666. https://doi.org/10.2135/cropsci2000.0009br
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14:1–16. https://doi.org/10.1079/SSR2003150
Bliss BJ, Wanke S, Barakat A (2013) Characterization of the basal angiosperm Aristolochia fimbriata: a potential experimental system for genetic studies. BMC Plant Biol 13:13. https://doi.org/10.1186/1471-2229-13-13
Che CT, Almed MS, Kang SS, Waller DP, Bengel AS, Martin A, Rajamahendran P, Bunyapraphatsara J, Lankin DC, Cordell GA, Soejarto DD, Wijesekera ROB, Fong HHS (1984) Studies on Aristolochia III: isolation and biological evaluation of constituents of Aristolochia indica roots for fertility-regulationg activity. J Nat Prod 47:331–341. https://doi.org/10.1021/np50032a017
Corner EJH (1976) The seeds of dicotyledons, vols 1 and 2. Cambridge University Press, Cambridge
Daws MI, Jennifer D, Pritchard HW, Brown NAC, Van Staden J (2007) Butenolide from plant-derived smoke enhances germination and seeding growth of arable weed species. Plant Grow Reg 51:73–82. https://doi.org/10.1021/np900630w
Daws MI, Pritchard HW, Van Staden J (2008) Butenolide from plant derived smoke functions as a strigolactone analogue: evidence from parasitic weed seed germination. South Afr J Bot 74:116–120. https://doi.org/10.1016/j.sajb.2007.09.005
Daws N, Van Staden J (2007) The potential of the smoke-derived compound 3-methyl-2H-furo [2,3-c] pyran-2-one as a priming agent for tomato seeds. Seed Sci Res 17:175–181. https://doi.org/10.1017/S0960258507785896
De Cuyper C, Struk S, Braem L, Gevaert K, De Jaeger G, Goormachtig S (2017) Strigolactones, karrikins and beyond. Plant Cell Env 40:1691–1703. https://doi.org/10.1111/pce.12996
De Lange JH, Boucher C (1990) Autecological studies on Audouinia capitata (Bruniaceae). I. Plant-derived smoke as a seed germination cue. South Afr J Bot 56:700–703. https://doi.org/10.1016/S0254-6299(16)31009-2
Drewes FE, Smith MT, Van Staden J (1995) The effect of a plant-derived smoke extract in the germination of light sensitive lettuce seed. Plant Grow Regul 16:205–209. https://doi.org/10.1007/BF00029542
Flematti GR, Ghisalberti EL, Dixon K, Trengrove RD (2004) A compound from smoke that promotes seed germination. Science 305:977. https://doi.org/10.1126/science.1099944
Flematti GR, Ghisalberti EL, Dixon KW (2005) Synthesis of the seed germination stimulant 3-methyl-2H-furo [2, 3-c] pyran-2-one. Tetr Lett 46:5719–5721. https://doi.org/10.1016/j.tetlet.2005.06.077
International Agency for Research on Cancer, Lyon (2002) Some traditional Herbal Medicines, some mycotoxins, Naphthalene and Styrene. IARC Monogram on evaluation of carcinogenic risks in humans. IARC. https://doi.org/10.1016/S0378-8741(03)00216-2
Izquierdo AM, Zapata EV, Jiménez-Ferrer JE, Muñoz CB, Aparicio AJ, Torres KB, Torres LO (2010) Scorpion antivenom effect of micropropagated Aristolochia elegans. Pharm Biol 48:891–896. https://doi.org/10.3109/13880200903311110
Jain N, Strik WA, Van Staden J (2008) Cytokinin-and auxin-like activity of a butenolide isolated from plant-derived smoke. South Afr J Bot 74:327–331. https://doi.org/10.1016/j.sajb.2007.10.008
Kubmarawa D, Ajoku GA, Enwerem NM, Okorie DA (2007) Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria. Afr J Biotechnol 6:1690–1696. https://doi.org/10.5897/AJB2007.000-2246
Kulkarni MG, Sparg SG, Light ME, Van Staden J (2006) Stimulation of rice (Oryza sativa L.) seedling vigour by smoke-water and butenolide (3-methyl-2H-furo [2, 3-c] pyran-2-one). J Agr Crop Sci 192:395–398. https://doi.org/10.1111/j.1439-037X.2006.00213.x
Lawrence MK, González F (2003) Phylogenetic relationships in Aristolochiaceae. Syst Bot 28:236–249. https://doi.org/10.1043/0363-6445-28.2.236
Long RL, Williams K, Griffiths EM, Flematti GR, Merritt DJ, Stevens JC, Turner SR, Powles SB, Dixon KW (2010) Prior hydration of Brassica tournefortii seeds reduces the stimulatory effect of karrikinolide on germination and increases seed sensitivity to abscisic acid. Ann Bot 105:1063–1070. https://doi.org/10.1093/aob/mcq061
Lopes LMX, Nascimento IR, da Silva T (2001) Phytochemistry of the Aristolochiaceae family. In: Mohan RMM (ed) Research advances in Phytochemistry, vol 2. Global Research Network, Kerala, pp 9–108
Luciano RL, Perazella MA (2015) Aristolochic acid nephropathy: epidemiology, clinical presentation, and treatment. Drug Saf 38:55–64. https://doi.org/10.1007/s40264-014-0244-x
Ma GH, Bunn E, Dixon K, Flemati G (2006) Comparative enhancement of germination and vigour in seed and somatic embryos by the smoke chemical 3-methyl-2H-furo[2,3-c] pyran-2-one in Baloskion tetraphyllum (Restionaceae). In Vitro Cell Dev Biol - Plant 42:305–308. https://doi.org/10.1079/IVP2006758
Maekawa L, Albuquerque MCF, Coelho MFB (2010) Germination of Aristolochia esperanzae O. Kuntze seeds under different temperatures and light conditions. Rev Bras Plant Med 12:23–30. https://doi.org/10.1590/S1516-05722010000100005
Mangnus ED, Zwanenburg B (1992) Tentative molecular mechanism for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogues. J Agric Food Chem 40:1066–1070. https://doi.org/10.1021/jf00018a032
Merritt DJ, Kristiansen M, Flematti GR, Turner SR, Ghisalberti EL, Trengove RD, Dixon KW (2006) Effects of a butenolide present in smoke on light-mediated germination of australian Asteraceae. Seed Sci Res 16:29–35. https://doi.org/10.1079/SSR2005232
Nakonechnaya OV, Gorpenchenko TY, Voronkova NM, Kholina AB, Zhuravlev YN (2013) Embryo structure, seed traits, and productivity of relict vine Aristolochia contorta (Aristolochiaceae). Flora 208:293–297. https://doi.org/10.1016/j.flora.2013.03.010
Nakonechnaya OV, Nesterova SV, Voronkova NM (2018) Germination of Aristolochia seeds (Aristolochiaceae). Mosc Univ Biol Sci Bull 73:209–216. https://doi.org/10.3103/S0096392518040077
Nedelko T, Arlt VM, Phillips DH, Hollstein M (2009) TP53 mutation signature supports involvement of aristolochic acid in the aetiology of endemic nephropathy-associated tumours. Int J Cancer 124:987–990. https://doi.org/10.1002/ijc.24006
Nelson DC, Flematti GR, Riseborough JA, Ghisalberti EL, Dixon KW, Steven MS (2010) Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc Nat Acad Sci USA 107:7095–7100. https://doi.org/10.1073/pnas.0911635107
Nelson DC, Riseborough JA, Flematti GR, Stevens J, Ghisalberti EL, Dixon K, Smith SM (2009) Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol 149:863–873. https://doi.org/10.1104/pp.108.131516
Ng AWT, Poon SL, Huang MN, Lim JQ, Boot A, Yu W, Suzuki Y, Thangaraju S, Ng CCY, Tan P, Pang ST, Huang HY, Yu MC, Lee PH, Hsieh SY, Chang AY, Teh BT, Rozen SG (2017) Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci Transl Med 18:eaan6446. https://doi.org/10.1126/scitranslmed.aan6446
Ribeiro LC, Pedrosa M, Borghetti F (2013) Heat shock effects on seed germination of five brazilian savanna species. Plant Biol 15:152–157. https://doi.org/10.1111/j.1438-8677.2012.00604.x
Roeder M, Ferraz IK, Hölscher D (2013) Seed and germination characteristics of 20 amazonian liana species. Plants 2:1–15. https://doi.org/10.3390/plants2010001
Rücker G, Mayer R, Breitmaier E, Will G, Kirfel A, Kordy ME (1984) Oxidized aristolane sesquiterpenes from. Aristolochia debilis Phytochemistry 23:1647–1649. https://doi.org/10.1016/S0031-9422(00)83460-3
Sami A, Rehman S, Ayyoub TM, Zhou XY, Zhu ZH, Zhou K (2021) Assessment of the germination potential of Brassica oleracea seeds treated with karrikin 1 and cyanide, which modify the ethylene biosynthetic pathway. J Plant Grow Reg 40:1257–1269. https://doi.org/10.1007/s00344-020-10186-1
Sharma G, Rajanna L (2021) GC-MS phytochemical profiling of leaf extracts of Aristolochia tagala cham. A rare and important ethnomedicinal plant. NISCAIR-CSIR, India. J Indian Chem Soc 100:100807. https://doi.org/10.1016/j.jics.2022.100807
Smith SM, Li J (2014) Signalling and responses to strigolactones and karrikins. Curr Opin Plant Biol 21:23–29. https://doi.org/10.1016/j.pbi.2014.06.003
Sparg SG, Kulkarni MG, Light ME, Van Staden J (2005) Improving seedling vigour of indigenous medicinal plants with smoke. Biores Tech 96:1323–1330. https://doi.org/10.1016/j.biortech.2004.11.015
Sulyman AO, Akolade JO, Sabiu SA, Aladodo RA, Muritala HF (2016) Antidiabetic potentials of ethanolic extract of Aristolochia ringens (vahl.) Roots. J Ethnopharmacol 182:122–128. https://doi.org/10.1016/j.jep.2016.02.002
Surhone LM, Tennoe MT, Henssonow SF (2011) Aristolochia labiata. Betascript Publishing, London
Van Staden J, Jäger AK, Strydom A (1995) Interaction between a plant-derived smoke extract, light and phytohormones on the germination of light-sensitive lettuce seeds. Plant Grow Regul 17:213–218. https://doi.org/10.1007/BF00024728
Voronkova NM, Kholina AB, Koldaeva MN, Nakonechnaya OV, Nechaev VA (2018) Morpho-physiological dormancy, germination, and cryopreservation in Aristolochia contorta seeds. Plant Ecol Evol 151:77–86. https://doi.org/10.5091/plecevo.2018.1351
Waldie T, McCulloch H, Leyser O (2014) Strigolactones and the control of plant development: lessons from shoot branching. Plant J 79:607–622. https://doi.org/10.1111/tpj.12488
Wu KM, Farrelly JG, Upton R, Chen J (2007) Complexities of the herbal nomenclature system in traditional chinese medicine (tcm): lessons learned from the misuse of Aristolochia-related species and the importance of the pharmaceutical name during botanical drug product development. Phytomedicine 14:273–279. https://doi.org/10.1016/j.phymed.2006.05.009
Wu TS, Damu AG, Su CR, Kuo PC (2004) Terpenoids of Aristolochia and their biological activities. Nat Prod Rep 21:594–624. https://doi.org/10.1002/chin.200504233
Yang T, Lian Y, Wang CY (2019) Comparing and contrasting the multiple roles of butenolide plant growth regulators: strigolactones and karrikins in plant development and adaptation to abiotic stresses. Intern J Mol Sci 20:6270. https://doi.org/10.3390/ijms20246270
Yao JR, Waters MT (2020) Perception of karrikins by plants: a continuing enigma. J Exp Bot 71:1774–1781. https://doi.org/10.1093/jxb/erz548
Yu JQ, Liao ZX, Cai XQ, Lei JC, Zouc GL (2007) Composition, antimicrobial activity and cytotoxicity of essential oils from Aristolochia mollissima. Environ Toxicol Pharmacol. https://doi.org/10.1016/j.etap.2006.08.004
Zhou J, Teixeira da Silva JA, Ma GH (2014) Effects of smoke water and karrikin on seed germination of 13 species growing in China. Centr Eur J Biol 9:1108–1116. https://doi.org/10.2478/s11535-014-0338-6
Zhou J, Xie G, Yan X (2011) Encyclopedia of Traditional Chinese Medicines: Molecular Structures, pharmacological activities, natural sources and applications. Springer, Berlin, Germany, pp 1–730. https://doi.org/10.1007/978-3-642-16744-7_1
Author Information
Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, The Chinese Academy of Sciences, Guangzhou, China