Enhancement of production of pharmaceutically important anti-cancerous compound; cucurbitacin E via elicitation and precursor feeding of in vitro culture of Citrullus colocynthis (L.) Schard
Dasari Ramakrishna, Gopu Chaitanya, Vankudoth Suvarchala, Dharavath Sunitha, Taduri Shasthree
Research Articles | Published: 03 April, 2020
First Page: 323
Last Page: 334
Views: 3711
Keywords: Citrullus colocynthis , Methyl jasmonate (MeJA), Salicylic acid (SA), Elicitors, Precursors, Cucurbitacin E, Squalene and mevalonic acid
Abstract
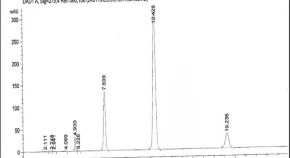
Citrullus colocynthis (L.) Schard (Cucurbitaceae) is an endangered medicinal herb. It contains glycosides, cucurbitacins (colocynthin and colocynthetin), cucurbitacins A, B, C, D, E, I, J, K and L which are having medicinal values in various parts of the plant. Cucurbitacin E, a highly oxidated steroid consisting of a tetracyclic triterpene which is more potential triterpenoid for anti-cancer. In this study, we made an attempt to investigate the effect of elicitors with methyl jasmonate (MeJA), salicylic acid (SA), precursors like squalene and mevalonic acid as abiotic elicitors. Precursors were used for enhancement production of cucurbitacin E through cell suspension culture and in vitro plant cultures of Citrullus colocynthis. Based on HPLC results, MeJA is better elicitor than SA and in precursors squalene is better than mevalonic acid for cucurbitacin E production from Citrullus colocynthis cell suspension and in vitro plant cultures. Among all the concentration of elicitors MeJA, SA and precursors squalene, mevalonic acid, 75 µM concentrations have shown best results and MeJA showed highest peak area of 17.97 with 8.63 fold in cell suspension culture. Whereas, the effect of elicitor and precursor feeding studies in in vitro plant culture have produced high amount of cucurbitacin E at 100 µM concentration. Hence, the protocol established in the present study provides an opportunity for enhancement of biological active (anticancer) compound cucurbitacin E.
References
- Ali M, Abbasi BH, Ali GS (2015) Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult 120:1099–1106
- Althawadi AM, Grace J (1986) Water use by the desert cucurbit Citrullus colocynthis (L.) Schrad. Oecologia 70:475–480
- Baldi A, Dixit V (2008) Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Biores Technol 99:4609–4614
- Bharate S, Gnana O (2006) Antidiabetic medicinal plants. In: Trivedi PC (ed) Medicinal plants ethnobotanical approach. Agrios Publisher, Jodhpur, pp 85–106
- Chodisetti B, Rao K, Gandi S, Giri A (2015) Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules methyl jasmonate and salicylic acid. Vitro Cell Dev Biol Plant 51:88–92
- Dasari R, Narra M, Ellandula R, Kota S, Taduri S (2015) Efficient in vitro propagation system via multiple shoot induction and assessment of clonal fidelity of regenerates in Citrullus colocynthis (L.) Schard. J Plant Cell Biotechnol Mol Biol 16(3 and 4):108–118
- Gurudeeban S, Satyavani K, Ramanathan T (2010) Bitter apple (Citrullus colocynthis): an overview of chemical composition and biomedical potentials. Asian J Plant Sci 9:394
- Katsaridis V, Papagiannaki C, Aimar E (2009) Embolization of brain arteriovenous malformations for cure: because we could and because we should. Am J Neuroradiol 30:e67
- Kirtikar K, Basu B (1987) Indian medicinal plants, vol 1–4. International Book Distributors, Dehradun
- Mustafa NR, De Winter W, Van Iren F, Verpoorte R (2011) Initiation, growth and cryopreservation of plant cell suspension cultures. Nat Protoc 6(6):715–742
- Patel H, Krishnamurthy R (2013) Elicitors in plant tissue culture. J Pharmacogn Phytochem 2(2):60–65
- Patel D, Prasad S, Kumar R, Hemalatha S (2012) An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed 2:320–330
- Patil JG, Ahire ML, Nitnaware KM, Panda S, Bhatt VP, Kishor PB, Nikam TD (2013) In vitro propagation and production of cardiotonic glycosides in shoot cultures of Digitalis purpurea L. by elicitation and precursor feeding. Appl Microbiol Biotechnol 97:2379–2393
- Ramakrishna D, Shasthree T (2016) High efficient somatic embryogenesis development from leaf cultures of Citrullus colocynthis (L.) Schard for generating true clones. Physiol Mol Biol Plants 22(2):279–285
- Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2):182
- Shabani L, Ehsanpour A, Asghari G, Emami J (2009) Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ J Plant Physiol 56:621–626
- Sivanandhan G, Arun M, Mayavan S, Rajesha M, Mariashibub TS, Manickavasagama M, Selvaraj N, Ganapathi A (2012) Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crops Prod 37:124–129
- Sivanandhan G, Kapil Dev G, Jeyaraj M, Rajesh M, Muthuselvam M, Selvaraj N, Manickavasagam M, Ganapathi A (2013) A promising approach on biomass accumulation and withanolides production in cell suspension culture of Withania somnifera (L.) Dunal. Protoplasma 250:885–898
- Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M (2014) An efficient hairy root culture system for Withania somnifera (L.) Dunal. Afr J Biotechnol 13(43):4141–4147
- Warrier P, Nambiar V, Ramankutty C (1995) Indian Medicinal Plants: a compendium of 500 species, vol 3. Orient Longman Pvt. Ltd., Chennai, pp 38–42
- Yadav J, Kumar S, Siwach P (2006) Folk medicine used in gynecological and other related problems by rural population of Haryana. Indian J Tradit knowl 5(3): 323–326
- Zhao T, Krokene P, Björklund N, Långström B, Solheim H, Christiansen E, Borg-Karlson AK (2010) The influence of Ceratocystis polonica inoculation and methyl jasmonate application on terpene chemistry of Norway spruce, Picea abies. Phytochemistry 71:1332–1341
- Zhong JJ (2001) Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. Adv Biochem Eng Biotechnol 72:1–26
Author Information
Plant Cell Tissue and Organ Culture Laboratory, Department of Biotechnology, Kakatiya University, Warangal, India