Enhancing vegetative growth and nutrient content of Gliricidia sepium (Jacq.) Kunth ex Walp using two Sri Lankan Bacillus species
*Article not assigned to an issue yet
Sandamini P. K. D., Mulec I. Mandić, Grabner E., Kumara H. W. K. S. L., Jayaprada N. V. T., Geekiyanage S.
Research Articles | Published: 05 December, 2025
First Page: 0
Last Page: 0
Views: 2
Keywords: n Bacillusn , n Gliricidia sepiumn , Nutrient content, Vegetative growth
Abstract
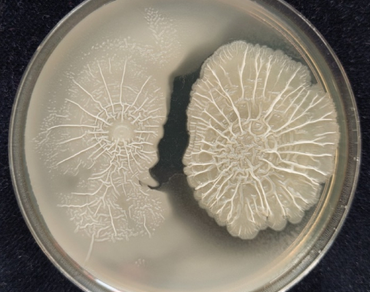
Inorganic fertilizer use poses environmental and health concerns despite the increased yields in commercial agriculture. Gliricidia sepium is a versatile plant in organic agriculture. Organic manure lacks sufficient amounts of readily available nutrients for plants. This study was conducted to determine the effect of two bacterial strains from Sri Lanka on enhancing the quantity and nutrient content of Gliricidia sepium for efficient utilization in sustainable agriculture. Two isolates I–I and I–II were partially identified based on rDNA and gyrA genes as Bacillus spp. and a phylogenetic tree was built. Plant growth-promoting attributes of phosphorus solubilization ability, Indole acetic acid production (IAA), and catalase activity were determined under laboratory conditions. A greenhouse experiment was conducted with Gliricidia sepium stem cuttings inoculated with two strains. Plant growth-promoting characters and nutrient contents in dry leaves were determined. The two strains were partially identified as Bacillus spp. with phosphorus solubilizing ability mainly. Plants inoculated with both strains enhanced plant height, leaf number, internodal length, SPAD values, root length, and dry shoot and root weights in contrast to control plants. Nodule formation was positively affected in inoculated plants. Nitrogen, phosphorus, and potassium levels in dry leaves, as well as nitrogen levels in pot soil, significantly increased in inoculated plants. Our findings concluded that the positive effect of two strains on Gliricidia sepium, which sheds light on their application in sustainable agriculture.
References
Ali AM, Awad MYM, Hegab SA, Gawad AMAE, Eissa MA (2020) Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J Plant Nutr 44(3):411–420. https://doi.org/10.1080/01904167.2020.1822399
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Amoo AE, Enagbonma BJ, Ayangbenro AS, Babalola OO (2021) Biofertilizer: an eco-friendly approach for sustainable crop production. In: Babalola OO (ed) Food security and safety. Springer, Cham, pp 647–669. https://doi.org/10.1007/978-3-030-50672-8_32
Bai Y, Zhou X, Smith DL (2003) Enhanced soybean plant growth resulting from co-inoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci 43:1774–1781. https://doi.org/10.2135/cropsci2003.1774
Belcijan Pandur K, Kraigher B, Tomac A, Stefanic P, Mandic Mulec I (2024) Nonkin interactions between Bacillus subtilis soil isolates limit the spread of swarming-deficient cheats. ISME J 18:wrae199. https://doi.org/10.1093/ismejo/wrae199
Chathurika LGI, Mandic-Mulec I, Greenberg EP, Senanayake G, Geekiyanage S (2024) Effect of two bacterial isolates on selected improved rice varieties under in vitro and greenhouse conditions. Trop Agric Res Ext 27:41–48. https://doi.org/10.4038/tare.v27i1.5699
Chun J, Bae KS (2000) Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek 78(2):123–127. https://doi.org/10.1023/a:1026555830014
Datta B, Chakrabartty PK (2014) Siderophore biosynthesis genes of Rhizobium sp. isolated from Cicer arietinum L. 3 Biotech 4(4):391–401. https://doi.org/10.1007/s13205-013-0164-y
Figueiredo CC, Moreira TN, Coser TR, Silva LP, Leite GG, de Carvalho AM, Malaquias JV, Marchão RL, Urquiaga S (2023) Nitrogen use efficiency in an agrisilviculture system with Gliricidia sepium in the Cerrado region. Plants 12:1647. https://doi.org/10.3390/plants12081647
Gang S, Sharma S, Saraf M, Buck M, Schumacher J (2019) Analysis of Indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-Protoc 9(9):e3230. https://doi.org/10.21769/BioProtoc.3230
Helgason E, Okstad OA, Caugant DA, Johansen HA, Fouet A, Mock M, Hegna I, Kolstø AB (2000) Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl Environ Microbiol 66(6):2627–2630. https://doi.org/10.1128/AEM.66.6.2627-2630.2000
Idris EE, Iglesias DJ, Talon M, Borriss R (2007) Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact 20(6):619–626. https://doi.org/10.1094/MPMI-20-6-0619
Jayasumana C, Fonseka S, Fernando A, Jayalath K, Amarasinghe M, Siribaddana S, Gunatilake S, Paranagama P (2015) Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springerplus 4:90. https://doi.org/10.1186/s40064-015-0868-z
John DA, Babu GR (2021) Lessons from the aftermaths of Green Revolution on food system and health. Front Sustain Food Syst 5:644559. https://doi.org/10.3389/fsufs.2021.644559
Kaba JS, Zerbe S, Agnolucci M, Scandellari F, Abunyewa AA, Giovannetti M, Tagliavini M (2019) Atmospheric nitrogen fixation by gliricidia trees (Gliricidia sepium (Jacq.) Kunth ex Walp.) intercropped with cocoa (Theobroma cacao L.). Plant Soil 435:323–336. https://doi.org/10.1007/s11104-018-3897-x
Khalifa AYZ, Almalki MA (2015) Isolation and characterization of an endophytic bacterium, Bacillus megaterium BMN1, associated with root-nodules of Medicago sativa L. growing in Al-Ahsaa region, Saudi Arabia. Ann Microbiol 65:1017–1026. https://doi.org/10.1007/s13213-014-0946-4
Kinkema M, Scott PT, Gresshoff PM (2006) Legume nodulation: successful symbiosis through short- and long-distance signalling. Funct Plant Biol 33:707–721. https://doi.org/10.1071/FP06056
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Kumvinit A, Akarapisan A (2020) Surfactin production, biofilm formation, and antagonistic activity against phytopathogens by Bacillus velezensis. Asian J Plant Pathol 14:27–35
Lastochkina O, Aliniaeifard S, Garshina D, Garipova S, Pusenkova L, Allagulova C, Fedorova K, Baymiev A, Koryakov I, Sobhani M (2021) Seed priming with endophytic Bacillus subtilis strain specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J Plant Physiol 263:153462. https://doi.org/10.1016/j.jplph.2021.153462
Menegat S, Ledo A, Tirado R (2022) Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci Rep 12:1–13. https://doi.org/10.1038/s41598-022-18773-w
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate-solubilizing microorganisms. FEMS Microbiol Lett 170:265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Olsen SR, Cole CV, Watanabe FS (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular No. 939. US Government Printing Office, Washington DC.
Oslizlo A, Stefanic P, Vatovec S, Beigot Glaser S, Rupnik M, Mandic-Mulec I (2015) QS diversity of Bacillus subtilis rhizoplane isolates. Microb Biotechnol 8:527–540. https://doi.org/10.1111/1751-7915.12258
Peter K, Zaharah A, Nguluu S (2020) Contribution of Gliricidia sepium pruning and fallow to sweet corn (Zea mays L. var. rugosa) yield, nitrogen uptake, release pattern, and use efficiency in a humid tropical environment of Malaysia. In: Advances in Agricultural and Biological Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-58065-0_16
Pramanik RS, Chandwani S, Amaresan N (2023) Screening of endophytes for plant growth-promoting metabolites. In: Sankaranarayanan A, Amaresan N, Dwivedi MK (eds) Endophytic microbes: isolation, identification, and bioactive potentials. Springer Protocols Handbooks, New York. https://doi.org/10.1007/978-1-0716-2827-0_19
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(D1):D590–D596. https://doi.org/10.1093/nar/gks12199
Reiner K (2010) Catalase test protocol. American society for microbiology. Available at: https://asm.org/getattachment/72a871fc-ba92-4128-a194-6f1bab5c3ab7/Catalase-Test-Protocol.pdf. Accessed 30 Dec 2023.
Sheteiwy M, AbdElgawad H, Xiong Y, Macovei A, Brestic M, Skalicky M, Shaghaleh H, Hamoud Y, El-Sawah A (2021) Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improves seed yield and quality of soybean plants. Physiol Plant 172:13454. https://doi.org/10.1111/ppl.13454
Sparks DL (ed) (1996) Methods of soil analysis: Part 3—chemical methods. Soil science society of America. WI, Madison
Subramanian P, Dhanapal R, Sanil P et al (2005) Gliricidia sepium as green manure in improving soil fertility and productivity of coconut under coastal littoral sandy soil. J Plant Crops 33:179–183
Tripathi N, Sapra A (2023) Gram staining. StatPearls Publishing, USA
Author Information
Faculty of Graduate Studies, University of Ruhuna, Matara, Sri Lanka