Ethnobotany, microscopy, phytoconstituents, and antioxidant activity of Xylosma longifolia Clos (Salicaceae) from Assam, India
*Article not assigned to an issue yet
Bhattacharyya Rakhi, Mehmud Selim, Sultana Sabrin, Kumari Sony
Research Articles | Published: 15 October, 2025
First Page: 0
Last Page: 0
Views: 96
Keywords: n Xylosma longifolian , Ethnobotany, Phytochemical, Natural antioxidant, Microscopic features
Abstract
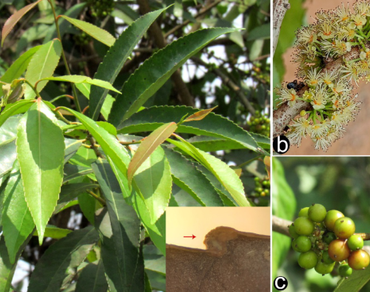
An ethnobotanical exploration among the tea garden communities in the Nagaon district of Assam (India) recorded the use of leaves of Xylosma longifolia against urinary tract infections and the fruits to facilitate parturition. The phytoconstituents of leaves and stem bark were previously investigated, but those of fruits, along with their antioxidant potential, remained unexplored. Furthermore, the microscopical features of this species were also unknown. To address these issues, plant samples were analyzed for microscopic study, including freehand transverse sections, epidermal morphology, and powder microscopy. A significant anatomical feature includes the presence of hypodermis in the leaf, leading to the first record for the genus. Through GC–MS analysis, the phytoconstituents of fruits were evaluated and recorded Pterin-6-carboxylic acid; 2(3H,4H)-Cyclopenta[b]furanone, 3a,6a-dihydro-; Limonene oxide, trans-; 3,4-Altrosan; D-mannoheptulose; Cyclohexanol, 1R-4-trans-acetamido-2,3-trans-epoxy; Methyl 9,10-octadecadienoate; Methyl 11-(2-cyclopenten-1-yl) undecanoate; and Ergosta-5, 22-dien-3-ol, acetate, (3ß,22E)-. The fruit extract demonstrates overall antioxidant property through DPPH assay, probably due to the presence of tannins and antioxidant compounds like Pterin-6-carboxylic acid, D-mannoheptulose, and Limonene oxide, trans-. The present study will contribute to the taxonomy of the genus, provide ethnobotanical information, and serve as a potential source for various biocompounds, natural antioxidants, and drug discovery.
References
Al-Ziaydi AG, Hamzah MI, Al-Shammari et al (2020) The anti-proliferative activity of D-mannoheptulose against breast cancer cell line through glycolysis inhibition. AIP Conf Proc 2307:020023. https://doi.org/10.1063/5.0032958
Ansari T, Saleem M, Asif M et al (2023) Morphological, phytochemical and ethnopharmacological attributes of Xylosma longifolia Closs: a review. J Pharmacogn Phytochem 21(1):679–689
Bain A, Sharma P, Kaur S et al (2023) Gum arabic/guar gum stabilized Hydnocarpus wightiana oil nanohydrolgel: characterization, antimicrobial, anti-inflammatory, and anti-biofilm activities. Int J Biol Macromol 239:124341. https://doi.org/10.1016/j.ijbiomac.2023.124341
Bhattacharyya R, Boruah JS, Medhi KK, Borkataki S (2020a) Phytochemical analysis of leaves of Xylosma longifolia Clos: a plant of ethnomedicinal importance. Int J Pharm Sci Res 11(5):2065–2074
Bhattacharyya R, Medhi KK, Borthakur SK, Borkataki S (2020b) An ethnobotanical study of medicinal plants used against jaundice by tea tribes of Morigaon district, Assam (India). J Nat Remedies 20(1):16–28
Bhattacharyya R, Mehmud S, Medhi KK, Borkataki S (2024) A report on ethnomedicinal plants used for the treatment of rheumatoid arthritis by the tea tribes of Morigaon district of Assam, India. Int J Ayurvedic Med 15(2):505–512. https://doi.org/10.47552/ijam.v15i2.4549
Bora D, Mehmud S, Das KK, Medhi H (2016) Report on folklore medicinal plant used for female health care in Assam (India). Int J Herb Med 4(6):04–13
Chamberlain CJ (1924) Methods in plant histology. The University of Chicago Press, Fourth Revised Edition
Chase MW, Zmartzty S, Lledó MD et al (2002) When in doubt, put it in Flacourtiaceae: a molecular phylogenetic analysis based on plastid rbcL DNA sequences. Kew Bull 57:141–181
Devi WR, Singh SB, Singh CB (2013) Antioxidant and anti-dermatophytic properties leaf and stem bark of Xylosma longifolium Clos. BMC Complement Altern Med 13:155
Duarte-Casar R, Romero-Benavides JC (2022) Xylosma G. Forst. Genus: medicinal and veterinary use, phytochemical composition, and biological activity. Plants 11:1252. https://doi.org/10.3390/plants11091252
GBIF (2025) Global Biodiversity Information Facility. www.gbif.org (accessed on August 2025)
Habila MM, Festus EA, Morumda D et al (2021) FTIR and GC-MS analysis of the aqueous and ethanolic extracts of Jatropha tanjorensis leaves. Open Sci J Biosci Bioeng 8(1):1–11
Hassan MM, Sharmila D, Nandini MS et al (2021) The GC MS analysis of ethyl acetate extract of one herbal plant ‘Ziziphus rugose.’ Nat Vol Essent Oil 8(4):6764–6771
Hussein AO, Mohammed GJ, Hadi MY, Hameed IH (2016) Phytochemical screening of methanolic dried galls extract of Quercus infectoria using gas chromatography-mass spectrometry (GC-MS) and Fourier transform-infrared (FT-IR). J Pharmacognosy Phytother 8(3):49–59. https://doi.org/10.5897/JPP2015.0368
Jackson BP, Snowdon DW (1990) Atlas of microscopy of medicinal plants, culinary herbs and spices. CBS Publishers and Distributors, New Delhi, India
Jadav V, Kalase V, Patil P (2014) GC-MS analysis of bioactive compounds in methanolic extract of Holigarna grahamii (wight) Kurz. Int J Herb Med 2(4):31–35
Judkevich MD, Salas RM, Gonzalez AM (2022) Anatomy and development of the edible fruits of Cordiera concolor (Rubiaceae). An Acad Bras Cienc 94(3):e20210071. https://doi.org/10.1590/0001-3765202220210071
Kanjilal UN, Kanjilal, PC, Das A et al (1934–1940) Flora of Assam. Volume 1–5. Government of Assam Press, Shillong.
Kekuda P, Onkarappa R, Gautham SA et al (2016) Antimicrobial, antioxidant and cytotoxic activity of Streptomyces species from Western Ghat soils of Karnataka. India Sci Technol Arts Res J 4:164. https://doi.org/10.4314/star.v4i2.20
Khare CP (2007) Indian medicinal plants: an illustrated dictionary. Springer-Verlag, Berlin Heidelberg, New York
Luo X, Su P, Zhang W (2015) Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar Drugs 13:4231–4254
Mao AA, Dash SS (2020) Flowering plants of India: an annotated checklist (Dicotyledons), vol Vol. 1. Printtech Offset Pvt. Ltd., Bhubaneswar
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons: leaves, stem, and wood in relation to taxonomy with notes on economic uses. Clarendon Press, Oxford
Oommen ST, Rao M, Raju CVN (1999) Effect of oil of Hydnocarpus on wound healing. Int J Lepr 67(2):154–158
Patial PK, Sharma A, Kaur I, Cannoo DS (2019) Correlation study among the extraction techniques, phytochemicals, and antioxidant activity of Nepetaspicta aerial part. Biocatal Agric Biotechnol 20:101275. https://doi.org/10.1016/j.bcab.2019.101275
POWO (2025) Plants of The World Online. https://powo.science.kew.org/ (accessed on August 2025)
Rejzková A, Vyskočilová E (2025) Limonene oxide isomerization: a broad study of acid catalyst effects. React Kinet Mech Catal 138:47–69. https://doi.org/10.1007/s11144-024-02774-z
Ruberto G, Baratta MT (2000) Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem 69:167–174. https://doi.org/10.1016/S0308-8146(99)00247-2
Sahoo MR, Dhanabal SP, Jadhav AN et al (2014) Hydnocarpus: an ethnopharmacological, phytochemical, and pharmacological review. J Ethnopharmacol 154:17–25. https://doi.org/10.1016/j.jep.2014.03.029
Sharma A, Bhardwaj G, Gaba J, Cannoo D (2020) Natural antioxidants: assay and extraction method/solvents used for their isolation. In: Nayik GA, Gull A (eds) Antioxidants in fruits: properties and health benefits. Springer Nature Singapore Pte. Ltd., Singapore, pp 1–34. https://doi.org/10.1007/978-981-15-7285-2
Shruthi CN, Kotresha D (2024) Chemical profiling and in vitro cytotoxic properties of Themeda triandra Forssk. against human bone osteosarcoma cell line (MG-63). Asian J Biol Life Sci 13(1):83–89
Swapana N, Noji M, Nishiuma R et al (2017) A new diphenyl ether glycoside from Xylosma longifolia collected from North-East India. Nat Prod Commun 12(8):1273–1275
Tesfay SZ, Bertling I, Bower JP (2012) D-mannoheptulose and perseitol in ‘Hass’ avocado: metabolism in seed and mesocarp tissue. S Afr J Bot 79:159–165. https://doi.org/10.1016/j.sajb.2011.10.006
Thadeo M, Cassino MF, Vitarelli NC et al (2008) Anatomical and histochemical characterization of extrafloral nectaries of Prockia crucis (Salicaceae). Am J Bot 95(12):1515–1522. https://doi.org/10.3732/ajb.0800120
Thadeo M, Azevedo AA, Meira RMSA (2014) Foliar anatomy of neotropical Salicaceae: potentially useful characters for taxonomy. Plant Syst Evol 300:2073–2089. https://doi.org/10.1007/s00606-014-1037-5
Truong BN, Pham VC, Mai HDT et al (2011) Chemical constituents from Xylosma longifolia and their anti-tubercular activity. Phytochem Lett 4:250–253. https://doi.org/10.1016/j.phytol.2011.04.008
Author Information
Department of Botany, Nowgong Girls’ College, Nagaon, India