Evaluation of root-to-shoot de novo organogenesis in wild guava species, Psidium schenckianum and P. guineense (Myrtaceae)
Research Articles | Published: 01 January, 2021
First Page: 68
Last Page: 76
Views: 4194
Keywords: De novo shoot organogenesis, Genetic stability, Guava, In vitro regeneration, Psidium
Abstract
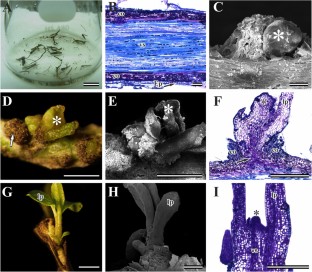
We aimed to evaluate the organogenic responses of root explants of Psidium schenckianum and P. guineense inoculated onto JADS liquid culture medium containing different concentrations (0.00, 2.22, and 4.44 µM) of 6-benzyladenine (BA) alone or in combination with 0.054 µM α-naphthaleneacetic acid (NAA). After 60 days of culture, the percentage of explants with shoots (PES) and number of shoots (NS) were recorded. Root explant samples were collected and analyzed under light and scanning electron microscopes. Elongated shoots were rooted and acclimatized. After acclimatization, the genetic stability of regenerated plants was characterized by flow cytometry. Shoot regeneration of P. guineense occurred at all treatments. On the other hand, the P. schenckianum did not respond at any conditions. The P. guineense species had the highest NS and PES at 2.22 and 0.00 µM BA, respectively, with no difference between these treatments. Shoots were formed via direct organogenesis from cells associated to the vascular tissues. Regenerated plants were acclimatized, yielding 100% survival rate. These regenerated plants kept the same ploidy as that of seed-germinated counterparts. This is the first report of P. guineense regeneration from root explants, which is a promising alternative for the micropropagation of this species.
References
- Abreu IS, Carvalho CR, Clarindo WR (2008) Chromosomal DNA content of sweet pepper determined by association of cytogenetic and cytometric tools. Plant Cell Rep 27:1227–1233
- Ali N, Mulwa RMS, Norton MA, Skirvin RM (2003) Micropropagation of guava (Psidium guajava L.). J Hortic Sci Biotechnol 78:739–741
- Atta R, Laurens L, Boucheron-Dubuisson E, Guivarch A, Carnero E et al (2009) Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyls explants grown in vitro. Plant J 57:626–644
- Balisteiro DM, Alezandro MR, Genovese MI (2013) Characterization and effect of clarified araçá (Psidium guineense Sw.) juice on postprandial glycemia in healthy subjects. Ciênc Tecnol Aliment 33:66–74
- Bassan JS, Reiniger LRS, Rocha BHG, Severo CRP, Flores AV (2006) Oxidação fenólica, tipo de explante e meios de cultura no estabelecimento in vitro de canafístula (Peltophorum dubium). Ciênc Florestal 16:381–390
- Bisen B, Bisen R, Singh Y (2014) Micropropagation of Guava (Psidium spp.): a review. Plant Arch 14:629–635
- Correia D, Gonçalves AN, Couto HYZ, Ribeiro MC (1995) Efeito do meio de cultura líquido e sólido no crescimento e desenvolvimento de gemas de Eucalyptus grandis×Eucalyptus urophylla na multiplicação in vitro. IPEF 48(49):107–116
- Coser SM, Ferreira MFS, Ferreira A, Mitre LK, Carvalho CR et al (2012) Assessment of genetic diversity in Psidium guajava L. using different approaches. Sci Hortic 148:223–229
- Costa IR, Dornelas MC, Forni-Martins ER (2008) Nuclear genome size variation in fleshy-fruited Neotropical Myrtaceae. Plant Syst Evol 276:209–217
- Cruz CD (2013) GENES—a software package for analysis in experimental statistics and quantitative genetics. Acta Sci Agron 35:271–276
- Cruz ACF, Rocha DI, Iarema L, Ventrella MC, Costa MGC et al (2014) In vitro organogenesis from root culture segments of Bixa orellana L. (Bixaceae). In Vitro Cell Dev Biol Plant 50:76–83
- De Smet I, Vanneste S, Inzé D, Beeckman T (2006) Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60:871–887
- Duclercq J, Sangwan-Norreel B, Catterou M, Sangwan RS (2011) De novo shoot organogenesis: from art to science. Trends Plant Sci 16:597–606
- Éder-Silva E, Felix LP, Bruno RDLA (2007) Citogenética de algumas espécies frutíferas nativas do nordeste do Brasil. Rev Bras Frutic 29:110–114
- Eng WH, Aziz MA, Sinniah UR (2015) In vitro regeneration of Citrus hystrix DC. Braz J Bot 38:235–242
- Erig AC, Schuch MW (2005) Tipo de luz na multiplicação in vitro de framboeseira (Rubus idaeus L.) ‘Batum.’ Rev Bras Frutic 27:488–490
- Galbraith DW (1989) Analysis of higher plants by flow cytometry and cell sorting. Int Rev Cytol 116:165–228
- González AMN, González MBR, Pinto NLS (2005) Estudio fitoquímico e actividad antibacterial de Psidium guineense Sw (choba) frente a Streptococcus mutans, agente causal de caries dentales. Rev Cubana Plant Med 1:3–4
- Guerra MP, Cangahuala-Inocente GC, Dal Vesco LL, Pescador R, Capestrano CA (2013) Micropropagation systems in Feijoa (Acca sellowiana (O. Berg.) Burret). In: Lambardi M et al (eds) Protocols for micropropagation of selected economically-important horticultural plants, methods in molecular biology, vol 994. Springer, New York
- Haminiuk CWI, Plata-Oviedo MSV, Guedes AR, Stafussa AP, Bona E et al (2011) Chemical, antioxidant and antibacterial study of Brazilian fruits. Int J Food Sci Tech 46:1529–1537
- Jain SM (2012) Date palm biotechnology: current status and prospective an overview. Emir J Food Agric 24:386–399
- Jani JN, Jha SK, Nagar DS (2015) Root explant produces multiple shoot from pericycle in Psoralea corylifolia—a leprosy destroyer medicinal plant. Ind Crops Prod 67:324–329
- Karnovsky MJA (1965) Formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
- Liu X, Yang G (2011) Clonal propagation of guava (Psidium guajava L) on nodal explants of mature elite cultivar. Int J Plant Biol 2:7–10
- Lombardi SP, Passos IRS, Nogueira MCS, Appezzato-da-Glória B (2007) In vitro shoot regeneration from roots and leaf discs of Passiflora cincinnata Mast. Braz Arch Biol Technol 50:239–247
- Lorenzi H, Bacher L, Lacerda M, Sartori S (2006) Frutas brasileiras e exóticas cultivadas (de consumo in natura). Instituto Plantarum de Estudos da Flora, São Paulo, 640 p
- Mallón R, Rodríguez-Oubina J, González ML (2011) Shoot regeneration from in vitro-derived leaf and root explants of Centaurea ultreiae. Plant Cell Tiss Organ Cult 106:523–530
- Manica I (2000) Frutas nativas, silvestres e exóticas 1: técnicas de produção e mercado: abiu, amora-preta, araçá, bacuri, biriba, carambola, cereja-do-rio-grande, jabuticaba. Cinco Continentes, Porto Alegre, 327 p
- Mazri MA (2015) Role of cytokinins and physical state of the culture medium to improve in vitro shoot multiplication, rooting and acclimatization of date palm (Phoenix dactylifera L.) cv. Boufeggous J Plant Biochem Biotechnol 24:268–275
- Motte H, Vereecke D, Geelen D, Werbrouck S (2014) The molecular path to in vitro shoot regeneration. Biotechnol Adv 32:107–121
- Nachtigal JC, Hoffmann A, Kluge RA, Fachinello JC, Mazzini ARA (1994) Enraizamento de estacas semilenhosas de araçazeiro (P. cattleyanum Sabine) com o uso do ácido indolbutírico. Rev Bras Frutic 16:229–235
- Nascimento KF, Moreira FMF, Santos JA, Kassuya CAL, Croda JHR et al (2018) Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J Ethnopharm 210:351–358
- O’Brien TP, McCully ME (1981) The study of plant structure principles and select methods. Termarcarphi Pty Ltd, Melbourne, 45 p
- Otto FJ (1990) DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Darzynkiewicz Z, Crissman HA (eds) Method in cell biology, vol 133. Academic Press, San Diego, pp 105–110
- Parveen S, Shahzad AA (2011) Micropropagation protocol for Cassia angustifolia Vahl. from root explants. Acta Physiol Plant 33:789–796
- Perilli S, Moubayidin L, Sabatini S (2010) The molecular basis of cytokinin function. Curr Opin Plant Biol 13:21–26
- Pessanha PGO, Viana AP, Amaral Júnior AT, Souza RM, Texeira MC et al (2011) Avaliação da diversidade genética em acessos de Psidium spp. via marcadores moleculares RAPD. Rev Bras Frutic 33:129–136
- Pilatti FK, Aguiar T, Simões T, Benson EE, Viana AM (2011) In vitro and cryogenic preservation of plant biodiversity in Brazil. In Vitro Cell Dev Biol Plant 47:82–98
- Rai MK, Jaiswal VS, Jaiswa U (2009) Shoot multiplication and plant regeneration of guava (Psidium guajava L.) from nodal explants of in vitro raised plantlets. J Fruit Ornam Plant Res 17:29–38
- Rai MK, Asthana P, Jaiswal U (2010) Biotechnological advances in guava (Psidium guajava L.): recent developments and prospects for further research. Trees 24:1–12
- Riefler M, Novak O, Strnad M, Schmülling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18:40–54
- Rocha DI, Vieira LM, Tanaka FAO, Silva LC, Otoni WC (2012) Anatomical and ultrastructural analyses of in vitro organogenesis from root explants of commercial passion fruit (Passiflora edulis Sims). Plant Cell Tiss Organ Cult 111:69–78
- Rocha DI, Vieira LM, Koehler AD, Otoni WC (2018) Cellular and morpho-histological foundations on in vitro plant regeneration. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Plant Cell Culture Protocols, Methods in Molecular Biology 1815:47–68
- Rodrigues CG, Ferreira PRB, Mendes CSO, Reis Junior R, Valerio HM et al (2014) Antibacterial activity of tannins from Psidium guineense Sw. (Myrtaceae). J Med Plants Res 8:1095–1100
- Rossi AAB, Clarindo WR, Carvalho CR, Oliveira LO (2008) Karyotype and nuclear DNA content of Psychotria ipecacuanha: a medicinal species. Cytologia 73:53–60
- Santos MAC, Queiroz MA, Santos AS, Santos LC, Carneiro PCS (2014) Diversidade genética entre acessos de araçá de diferentes municípios do semiárido baiano. Rev Caatinga 27:48–57
- Santos MAC, Rêgo MM, Queiróz MA, Caproni DTR, Dietrich OHS, Santos AF, Rocha DIR, Batsita DS, Otoni WC (2020) In vitro growth performance of Psidium guajava and P. guineense plantlets as affected by culture medium formulations. Vegetos 33:435–445
- Santos MAC, Queiroz MA, Bispo JDS, Dantas BF (2015) Seed germination of Brazilian guava (Psidium guineense Swartz.). J Seed Sci 37:214–221
- Santos MAC, Rego MM, Queiroz MA, Dantas BF, Otoni WC (2016) Synchronizing in vitro germination of Psidium guineense Sw. seeds by means of osmotic priming. Rev Árvore 40:649–660
- Shah ST, Zamir R, Ahmad J, Ali H, Lutfullah G (2008) In vitro regeneration of plantlets from seedling explants of Guava (Psidium guajava L.) cv. Safeda. Pakistan J Bot 40:1195–1200
- Silva JD, Luz AIR, Silva MHL, Andrade EHA, Zoghbi MGB et al (2003) Essential oils of the leaves and stems of four Psidium spp. Flavour Fragrance J 18:240–243
- Silva CV, Oliveira LS, Loriato VAP, Silva LC, Campos JMS et al (2011) Organogenesis from root explants of commercial populations of Passiflora edulis Sims and a wild passionfruit species, P. cincinnata Masters. Plant Cell Tiss Organ Cult 107:407–416
- Simões C, Albarello N, Callado CH, Castro TC, Mansur E (2009) New approaches for shoot production and establishment of in vitro root cultures of Cleome rosea Vahl. Plant Cell Tiss Organ Cult 98:79–86
- Singh SK, Meghwal PR, Sharma HC, Singh SP (2002) Direct shoot organogenesis on hypocotyl explants from in vitro germinated seedlings of Psidium guajava L. cv. Allahabad Safeda. Sci Hortic 95:213–221
- Souza AG, Resende LV, Lima IP, Martins LSS, Techio VH (2015) Chromosome number and nuclear DNA amount in Psidium spp. resistant and susceptible to Meloidogyne enterolobii and its relation with compatibility between rootstocks and commercial varieties of guava tree. Plant Syst Evol 301:231–237
- Su YH, Liu YB, Zhang XS (2011) Auxin-cytokinin interaction regulates meristem development. Mol Plant 4:616–625
- Sugimoto K, Jiao Y, Meyerowitz EM (2010) Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell 18:463–471
- Sugimoto K, Gordon SP, Meyerowitz EM (2011) Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol 21:212–218
- Usman M, Butt M, Fatima B (2012) Enhanced in vitro multiple shoot induction in elite Pakistani guava cultivars for efficient clonal plant multiplication. Afr J Biotechnol 11:10182–10187
- Van Staden J, Zazimalova E, George EF (2008) Plant growth regulators II: cytokinins, their analogues and antagonists. In: George EF, Hall MA, Klerk GJ (eds) Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 205–226
- Vieira LM, Rocha DI, Taquetti MF, Silva LC, Campos JMS et al (2014) In vitro plant regeneration of Passiflora setacea D.C. (Passifloraceae): the influence of explant type, growth regyulators, and incubation conditions. In Vitro Cell Dev Biol Plant 50:738–745
- Vila S, Gonzalez A, Rey H, Mroginski L (2005) Plant regeneration, origin, and development of shoot buds from root segments of Melia azedarach L. (Meliaceae) seedlings. In Vitro Cell Dev Biol Plant 41:746–751
- Yumbla-Orbes M, Cruz ACF, Pinheiro MVM, Rocha DI, Batista DS et al (2017) Somatic embryogenesis and de novo shoot organogenesis can be alternatively induced by reactivating pericycle cells in Lisianthus (Eustoma grandiflorum (Raf.) Shinners) root explants. In Vitro Cell Dev Biol Plant 53:209–218
Author Information
Centro de Ciências Agrárias, Universidade Federal da Paraíba, Campus II, Areia, Brazil