Evaluation of stigma receptivity and its properties in Helianthus annuus L. (Asteraceae)
Research Articles | Published: 06 July, 2022
First Page: 474
Last Page: 483
Views: 3481
Keywords: Antimicrobial, Esterases, Flavonoids, Peroxidases, Stigma
Abstract
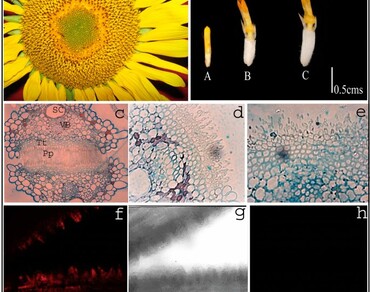
Stigma receptivity has a critical role in the development of flowering plants. The time of stigma receptivity is important as it results in pollen recognition, adhesion and formation of pollen tube. The duration of receptivity may vary from few hours to few days. At receptivity, the stigma accumulates different biomolecules required for reproductive success. The purpose of present investigation is to identify the biomolecules and assess their critical role, in receptivity of stigma. Receptivity of Helianthus annuus L. (family Asteraceae), is evaluated by activity of enzymatic markers (through enzymatic method for histochemical localization), and the associated signaling molecules (localized by confocal microscopy). Esterases and Peroxidases act as the marker enzymes for receptivity in flowering plants. Interplay of reactive oxygen species (ROS), hydrogen peroxides and flavonoids facilitate stigma to override the stressful environmental conditions and also provide it resistance against pathogens. Present investigations, further reveal that the secondary metabolites present in stigma of sunflower act efficiently against the gram negative (Escherichia coli) and gram positive (Staphylococcus spp.) bacteria thereby highlighting stigma’s ability to defend itself and inflorescence as a whole, from microbial infection during its development. The study, thus highlights the interplay of enzymes, reactive oxygen species and flavonoids in increasing cross-tolerance in stigma of H. annuus.
References
Allen AM, Thorogood CJ, Hegarty MJ, Lexer C, Hiscock SJ (2011) Pollen-pistil interactions and self-incompatibility in Asteraceae: new insights from studies of Senecio squalidus (Oxford ragwort). Ann Bot 108:687–698
Baroncelli S, Lercari B, Cecconi F, Pugliesi C (1990) Light control of elongation of filament in sunflower (Helianthus annuus L.). Photochem Photobiol 52:229–231
Bednarska E, Lenartowska M, Niekraś L (2005) Localization of pectins and Ca2+ ions in unpollinated and pollinated wet (Petunia hybrida Hort.) and dry (Haemanthus albiflos L.) stigma. Folia Histochem Cytobiol 43:249–259
Beltramo C, Marinoni DT, Perrone I, Botta R (2012) Isolation of a gene encoding for a class III peroxidase in female flower of Corylus avellana L. Mol Biol Rep 39:4997–5008
Beneš K (1968) On the stainability of plant cell walls with alcian blue. Biol Plant 10:334–346
Bhatla SC, Gogna M, Jain P, Singh N, Mukherjee S, Kalra G (2021) Signaling mechanisms and biochemical pathways regulating pollen-stigma interaction, seed development and seedling growth in sunflower under salt stress. Plant Signal Behav 16(11):1958129. https://doi.org/10.1080/15592324.2021.1958129
Bílková J, Albrechtová J, Opatrná J (1999) Histochemical detection and image analysis of non-specific esterase activity and the amount of polyphenols during annual bud development in Norway spruce. J Exp Bot 50:1129–1138
Brunetti C, Ferdinando MD, Fini A, Pollastri S, Tattini M (2013) Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci 14:3540–3555
Chen J, Gutjahr C, Bleckmann A, Dresselhaus T (2015) Calcium signaling during reproduction and biotrophic fungal interactions in plants. Mol Plant 8(4):595–611
Chen Z, Raji M (2020) Role of reactive oxygen species in modulating cross tolerance in plants via flavonoids. In: Hossain MA, Liu F, Burritt DJ, Fujita M, Huang M (eds) Priming-mediated stress and cross-stress tolerance in crop plants. Academic Press, ISBN 9780128178928
Cosio C, Dunand C (2009) Specific functions of individual class III peroxidase genes. J Exp Bot 60:391–408
Dafni A, Maués MM (1998) A rapid and simple procedure to determine stigma receptivity. Sex Plant Reprod 11:177–180
de Graff BHJ, Derksen JWM, Mariani C (2001) Pollen and pistil in the programic phase. Sexual Plant Reprod 14:411–455
Dickinson H (1995) Dry stigmas, water and self-incompatibility in Brassica. Sexual Plant Reprod 8:1–10
Dudek B, Warskulat AC, Schneider B (2016) The occurrence of flavonoids and related compounds in flower sections of Papaver nudicaule. Plants (basel) 5(2):28. https://doi.org/10.3390/plants5020028
Fábián A, Sáfrán E, Szabó-Eitel G, Barnabás B, Jäger K (2019) Stigma functionality and fertility are reduced by heat and drought co-stress in wheat. Front Plant Sci 10:244
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Amer J Bot 55:123–142
Galen C, Plowright RC (1987) Testing the accuracy of using peroxidase activity to indicate stigma receptivity. Can J Bot 65:107–111
Ghosh S, Shivanna KR (1980) Pollen-pistil interaction in Linum grandiflorum: stigma-surface proteins and stigma receptivity. Proc Indian Natl Sci Acad B46:177–183
Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR (2018) Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol 9:1639
Górniak I, Bartoszewski R, Króliczewski J (2019) Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 18:241–272
Heslop-Harrison Y, Shivanna KR (1977) The receptive surface of angiosperm. Ann Bot 41:1233–1258
Hiscock SJ, Hoedemaekers K, Friedman WE, Dickinson HG (2002) The stigma surface and pollen-stigma interactions in Senecio squalidus l. (Asteraceae) following cross (compatible) and self (incompatible) pollinations. Int J Plant Sci 163:1–16
Hong L, Shen H, Ye W, Cao H, Wang Z (2008) Secondary pollen presentation and style morphology in the invasive weed Mikania micrantha in South China. Bot Stud 49:253–260
Howell GJ, Slater AT, Knox RB (1993) Secondary pollen presentation in angiosperms and its biological significance. Aust J Bot 41:417–438
Hsieh K, Huang AH (2007) Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 19:582–596
Kandasamy MK, Kristen U (1987) Developmental aspects of ultrastructure, histochemistry and receptivity of the stigma of Nicotiana sylvestris. Ann Bot 60:427–437
Koning RE (1983) The roles of plant hormones in style and stigma growth in Gaillardia grandiflora (Asteraceae). Amer J Bot 70:978–986
Kulloli SK, Ramasubbu R, Sreekala AK, Pandurangan AG (2010) Cytochemical localization of stigma-surface esterases in three species of Impatiens (Balsaminaceae) of western ghats. Asian J Exp Biol Sci 1:106–111
Mattsson O, Knox RB, Heslop-Harrison J, Heslop-Harrison Y (1974) Protein pellicle of stigmatic papillae as a probable recognition site in incompatibility reactions. Nature 247:298–300
McInnis SM, Costa LM, Gutiérrez-Marcos JF, Henderson CA, Hiscock SJ (2005) Isolation and characterization of a polymorphic stigma-specific class III peroxidase gene from Senecio squalidus L. (Asteraceae). Plant Mol Biol 57:659–677
McInnis SM, Desikan R, Hancock JT, Hiscock SJ (2006a) Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytol 172:221–228
McInnis SM, Emery DC, Porter R, Desikan R, Hancock JT, Hiscock SJ (2006b) The role of stigma peroxidases in flowering plants: insights from further characterization of stigma-specific peroxidase (SSP) from Senecio squalidus (Asteraceae). J Exp Bot 57:1835–1846
Onus AN (2000) Structure of the stigma and style in Capsicum eximium and the effects of pollination. Turk J Bot 24:337–346
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540
Perez IB, Brown PJ (2014) The role of ROS signaling in cross-tolerance: from model to crop. Front Plant Sci 5:754
Polveiro RC, Vidigal PMP, Mendes TAO et al (2020) Effects of enrofloxacin treatment on the bacterial microbiota of milk from goats with persistent mastitis. Sci Rep 10:4421
Pruski JF, Sancho G (2004) Asteraceae or Compositae (Aster or Sunflower Family). In: Smith N et al (eds) Flowering plants of the neotropics. Princeton Univ. Press, Princeton
Quinn PJ, Carter ME, Markey B, Carter GR (1994) Clinical veterinary microbiology. Wolf/Mosby, London
Reger BJ (1989) Stigma surface secretions of Pennisetum americanum. Amer J Bot 76:1–5
Sammataro D, Garment MB, Erickson Jr EH (1985) Anatomical features of the sunflower floret. Helia (FAO, Romania) 25–31
Sanchez AM, Bosch M, Bots M, Nieuwland J, Feron R, Mariani C (2004) Pistil factors controlling pollination. Plant Cell 16:S98–S106
Schneiter AA, Miller JF (1981) Description of sunflower growth stages. Crop Sci 21:901–903
Shakya R (2020) Stigma receptivity with pollen in sunflower accompanies novel histochemical and biochemical changes in both male and female reproductive structures. Vegetos 33:376–384. https://doi.org/10.1007/s42535-020-00118-5
Sharma B (2019) An analyses of flavonoids present in the inflorescence of sunflower. Braz J Bot 42:421–429
Sharma B, Bhatla SC (2013a) Accumulation and scavenging of reactive oxygen species and nitric oxide correlate with stigma maturation and pollen-stigma interaction in sunflower. Acta Physiol Planta 35:2777–2787
Sharma B, Bhatla SC (2013b) Structural analysis of stigma development in relation with pollen-stigma interaction in sunflower. Flora 208:420–429
Sharma B, Bhatla SC (2014) Elemental and biochemical markers of stigma receptivity in sunflower. Acta Physiol Planta 36:1299–1311
Sharma B, Shakya R, Bhatla SC (2014) Floral biology of Sunflowers: a histological and physiological analysis. In: Arribas J (ed) Growth and development, environmental influences and pests/diseases. Nova, New York, pp 19–42
Shivanna KR, Rangaswamy NS (1992) Pollen biology: a laboratory manual. Springer-Verlag, Berlin
Teixeira SP, Capucho LC, Machado SR (2011) Two novel reports of semidry stigmatic surface in Asteraceae. Flora 206:328–333
Torres C, Galetto L (2007) Style morphological diversity of some Asteraceae species from Argentina: systematic and functional implications. J Plant Res 120(3):359–364
Verma H, Rawat S, Sharma N, Jaiswal V, Singh RB (2018) Prevalence, bacterial etiology and antibiotic susceptibility pattern of bovine mastitis in Meerut. J Entomol Zool Stud 6:706–709
Wu H-C, Bulgakov VP, Jinn T-L (2018) Pectin methylesterases: cell wall remodeling proteins are required for plant response to heat stress. Front Plant Sci 9:1612. https://doi.org/10.3389/fpls.2018.01612
Xingguo L, Jia Y, Kumar A, Yuzhe N, Xiaoyu L, Yuhua L, Marcus AS (2017) Flavonoids and ROS play opposing roles in mediating pollination in ornamental Kale (Brassica oleracea var. acephala). Mol Plant 10:1361–1364
Zhang MJ, Zhang XS, Gao XQ (2020) ROS in the male–female interactions during pollination: function and regulation. Front Plant Sci 11:177
Author Information
Department of Botany, Multanimal Modi College, Modinagar, India