Extensive studies on physiochemical properties, purification strategies, application of microbial l-amino acid oxidase
Yadav Monika, Taliyan Shivam Kumar, Kumar Ashok, Singh Priyanka
Review Articles | Published: 20 January, 2023
First Page: 38
Last Page: 51
Views: 3500
Keywords: l-Amino acid oxidase, Chromatographic methods, Polymeric phase system
Abstract
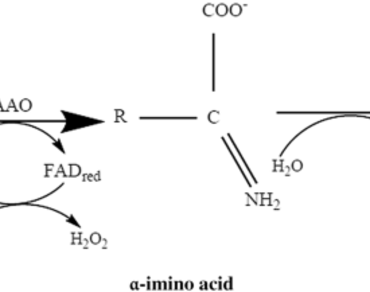
l-Amino acid oxidase (LAAO) as flavoenzymes catalyzes the stereospecific oxidative deamination of l-amino acids to α-keto acid along with the production of hydrogen peroxide and ammonia. This enzyme is widely distributed in bacteria, fungi, and mammals. LAAO from snake venom is widely studied for its potential to induce apoptosis, hemorrhage, aggregate platelets, and edema. The conventional bioseparation method of chromatographic techniques are generally reported for purification of this enzyme. It has major limitation of time consumption and high cost due to involvement of several purification strategies and equipment. Currently, liquid–liquid extraction processes like aqueous two-phase systems (ATPs) and three phase system (TPP) are being employed to overcome the problems associated with conventional multistep purification processes. Both ATPs and TPP are effectively used for purification of biomolecules because of their versatility, biocompatibility, rapid, easily scalable, energy efficient and relatively low cost attributes. ATPs has been used for a separation of soluble biomolecules such as proteins, amino acids, nucleic acids, plant and animal cells products, microbial products. In this review article, we have studied the physiological properties of different types of l-Amino acid oxidase. The downstream processing techniques for efficient extraction of these l-amino acid oxidases has been subsequently studied in terms of conventional chromatographic purification techniques and polymeric phase systems. This polymeric phase system based biosepration technique could be effectively used for recovery of l-Amino acid oxidase at higher extent. These purified extract of LAAO could be efficiently used with higher efficacy in different industrial sectors.
References
Abbot NL, Blankschtein D, Hatton TA (1990) On protein partitioning in two-phase aqueous polymer systems. Bioseparation 1:191–225
Abdelkafi-Koubaa Z, Aissa I, Morjen M, Kharrat N, El Ayeb M, Gargouri Y et al (2016) Interaction of a snake venom L-amino acid oxidase with different cell types membrane. Int J Biol Macromol 82:757–764. https://doi.org/10.1016/j.ijbiomac.2015.09.065
Abe Y, Shimoyama Y, Munakata H, Ito J, Nagata N, Ohtsuki K (1998) Characterization of an apoptosis-inducing factor in Habu snake venom as a glycyrrhizin (GL)-binding protein potently inhibited by GL in vitro. Biol Pharm Bull 21(9):924–927. https://doi.org/10.1248/bpb.21.924
Albertsson PA (1986) Partition of cell particles and macromolecules. Wiley (Interscience), New York
Ali SA, Stoeva S, Abbasi A, Alam JM, Kayed R, Faigle M, Voelter W (2000) Isolation, structural, and functional characterization of an apoptosis-inducing L-amino acid oxidase from leaf-nosed viper (Eristocophis macmahoni) snake venom. Arch Biochem Biophys 384(2):216–226
Alves RM, Antonucci GA, Paiva HH, Cintra ACO, Franco JJ, Mendonça-Franqueiro EP, Sampaio SV (2008) Evidence of caspase-mediated apoptosis induced by L-amino acid oxidase isolated from Bothrops atrox snake venom. Comp Biochem Physiol a: Mol Integr Physiol 151(4):542–550
Andrews BA, Pyle DL, Asenjo JA (1994) The effects of pH and ionic strength on the partitioning of four proteins in reverse micelle systems. Biotechnol Bioeng 43(11):1052–1058
Arima J, Sasaki C, Sakaguchi C, Mizuno H, Tamura T, Kashima A et al (2009) Structural characterization of L-glutamate oxidase from Streptomyces sp. X-119–6. FEBS J 276(14):3894–3903. https://doi.org/10.1111/j.1742-4658.2009.07103.x
Asenjo JA, Andrews BA (2011) Aqueous two-phase systems for protein separation: a perspective. J Chromatogr A 1218:8826–8835
Banik RM, Santhiagu A, Kanari B, Sabarinath C, Upadhyay SN (2003) Technological aspects of fermentation using aqueous two-phase systems. World J Microbiol Biotechn 19:337–348
Blanchard M, Green DE, Nocito V, Ratner S (1944) L-Amino acid oxidase of animal tissue. J Biol Chem 155:421–440
C, Nonato MC, de Albuquerque S, Ho PL, de Azevedo ILJ, Diniz MRV, Lomonte B, Rucavado A, Díaz C, Gutiérrez JM, Arantes EC (2012) Isolation and biochemical, functional and structural characterization of a novel L-amino acid oxidase from Lachesismuta snake venom. Toxicon 60(7):1263–1276
Chaiwut P, Pintathong P, Rawdkuen S (2010) Extraction and three phase partitioning behaviour of proteases from papaya peels. Process Biochem 45:1172–1175
Cheng C-H, Yang C-A, Liu S-Y, Lo C-T, Peng K-C (2012) L-Amino acid oxidase-induced apoptosis in filamentous Botrytis cinerea. Anal Biochem 420:93–95. https://doi.org/10.1016/j.ab.2011.09.003
Costa TR, Menaldo DL, Zoccal KF, Burin SM, Aissa AF, Castro FA et al (2017) CR-LAAO, an L-amino acid oxidase from Calloselasma rhodostoma venom, as a potential tool for developing novel immunotherapeutic strategies against cancer. Sci Rep 7:42673. https://doi.org/10.1038/srep42673
Costa Torres AF, Dantas RT, Toyama MH, Diz Filho E, Zara FJ, de QueirozaNadia MGR et al (2010) Antibacterial and antiparasitic effects of Bothrops marajoensis venom and its fractions: phospholipase A2 and l-amino acid oxidase. Toxicon 55(4):795–804. https://doi.org/10.1016/j.toxicon.2009.11.013
Costal-Oliveira F, Stransky S, Guerra-Duarte C, Naves de Souza DL, VivasRuiz DE, Yarlequé A et al (2019) L-amino acid oxidase from Bothrops atrox snake venom triggers autophagy, apoptosis and necrosis in normal human keratinocytes. Sci Rep 9(1):781
Davis MA, Askin MC, Hynes MJ (2005) Amino acid catabolism by an areA-regulated gene encoding an L-amino acid oxidase with broad substrate specificity in Aspergillus nidulans. Appl Environ Microbiol 71(7):3551–3555
Dennison C, Lovrien R (1997) Three phase partitioning: concentration and purification of proteins. Protein Expres Purif 11:149–161
Dhananjay SK, Mulimani VH (2009) Three phase partitioning of α-galactosidase from fermented media of Aspergillus oryzae and comparison with conventional purification techniques. J Ind Microbiol Biot 36:123–128
Du XY, Clemetson KJ (2002) Snake venom L-amino acid oxidases. Toxicon 40:659–665. https://doi.org/10.1016/S0041-0101(02)00102-2
Duman Y, Kaya E (2013) Purification, recovery and characterization of chick pea (Cicer arietinum) β-galactosidase in single step by three phase partitioning as a rapid and easy technique. Protein Expres Purif 91:155–160
Dypbukt JM, Ankarcrona M, Burkitt M, Sjoholm A, Strom K, Orrenius S, Nicotera P (1994) Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J Biol Chem 269(48):30553–30560
El-Sayed AS, Shindia AA, Zaher Y (2012) L-Amino acid oxidase from filamentous fungi: screening and optimization. Ann Microbiol 62(2):773–784
Feliciano PR, Rustiguel JK, Soares RO, Sampaio SV, Cristina Nonato M (2017) Crystal structure and molecular dynamics studies of L-amino acid oxidase from Bothrops atrox. Toxicon 15(128):50–59. https://doi.org/10.1016/j.toxicon.2017.01.017
Fernandez-Gomez R, Zerrouk H, Sebti F, Loyens M, Benslimane A, Ouaissi MA (1994) Growth inhibition of Trypanosoma cruzi and Leishmania donovani infantum by different snake venoms: preliminary identification of proteins from Cerastes cerastes venom which interact with the parasites. Toxicon 32(8):875–882
Findrik Z, Geueke B, Hummel W, Vasic-Racki Đ (2006) Modelling of L-DOPA enzymatic oxidation catalyzed by L-amino acid oxidases from Crotalus adamanteus and Rhodococcus opacus. Biochem Eng J 27(3):275–286
Gaur R, Sharma A, Khare SK, Gupta MN (2007) A novel process for extraction of edible oils-enzyme assisted three phase partitioning. Bioresource Technol 98:696–699
Georgieva D, Murakami M, Perband M, Arni R, Betzel C (2011) The structure of a native l-amino acid oxidase, the major component of the Vipera ammodytes ammodytes venomic, reveals dynamic active site and quaternary structure stabilization by divalent ions. Mol Biosyst 7(2):379–384. https://doi.org/10.1039/C0MB00101E
Guo C, Liu S, Yao Y, Zhang Q, Sun MZ (2012) Past decade study of snake venom L-amino acid oxidase. Toxicon 60(3):302–311
Harde SM, Singhal RS (2012) Extraction of forskolin from Coleus forskohlii roots using three phase partitioning. Sep Purif Technol 96:20–25
Hatti-Kaul R (ed) (2000) Aqueous two-phase systems: methods and protocols. methods in biotechnology, vol 11. Humana Press, New Jersey
Hossain GS, Li J, Shin HD, Du G, Liu L, Chen J (2014) L-Amino acid oxidases from microbial sources: types, properties, functions, and applications. Appl Microbiol Biotechnol 98(4):1507–1515
Huang YL, Li M, Yu Z, Qian PY (2011) Correlation between pigmentation and larval settlement deterrence by Pseudoalteromonas sp. sf57. Biofouling 27(3):287–293
Huddleston J, Veide A, Kohler K, Flanagan J, Enfors SO, Lyddiat A (1991) The molecular basis of partitioning in aqueous two-phase systems. Trends Biotechnol 9:381–388
Isobe K, Fukuda N, Nagasawa S (2008) Analysis of selective production of Nα-Benzyloxycarbonyl-l-Aminoadipate-o-Semialdehyde and Nα-Benzyloxycarbonyl-l-Aminoadipic Acid by Rhodococcus sp. AIU Z-35–1. J Biosci Bioeng 105(2):152–156
Isobe K, Sugawara A, Domon H, Fukuta Y, Asano Y (2012) Purification and characterization of an l-amino acid oxidase from Pseudomonas sp. AIU 813. J Biosci Bioeng 114(3):257–261
Izidoro LFM, Ribeiro MC, Souza GR, Sant’Ana CD, Hamaguchi A, Homsi-Brandeburgo MI, Rodrigues VM (2006) Biochemical and functional characterization of an L-amino acid oxidase isolated from Bothrops pirajai snake venom. Bioorganic Med Chemi 14(20):7034–7043
Izidoro LFM, Sobrinho JC, Mendes MM, Costa TR, Grabner AN, Rodrigues VM, Soares AM (2014) Snake venom L-amino acid oxidases: trends in pharmacology and biochemistry. BioMed Res Int 2014:196754. https://doi.org/10.1155/2014/196754
Kasai K, Ishikawa T, Komata T, Fukuchi K, Chiba M, Nozaka H et al (2010) Novel L-amino acid oxidase with antibacterial activity against methicillin-resistant Staphylococcus aureus isolated from epidermal mucus of the flounder Platichthys stellatus. FEBS J 277(2):453–465. https://doi.org/10.1111/j.1742-4658.2009.07497.x
Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy. Cancer 73(8):2013–2026
Kulkarni VM, Rathod VK (2014) Extraction of mangiferin from Mangifera indica leaves using three phase partitioning coupled with ultrasound. Ind Crop Prod 52:292–297
Kurmudle NN, Bankar SB, Bajaj IB, Bule MV, Singhal RS (2011) Enzyme assisted three phase partitioning: a novel approach for extraction of turmeric oleoresin. Process Biochem 46:423–426
Lazo F, Vivas-Ruiz DE, Sandoval GA, Rodríguez EF, Kozlova EEG, Costal-Oliveira F et al (2017) Biochemical, biological and molecular characterization of an L-Amino acid oxidase (LAAO) purified from Bothrops pictus Peruvian snake venom. Toxicon 1(139):74–86. https://doi.org/10.1016/j.toxicon.2017.10.001
Lee ML, Fung SY, Chung I, Pailoor J, Cheah SH, Tan NH (2014) King Cobra (Ophiophagus hannah) venom l-amino acid oxidase induces apoptosis in pc-3 cells and suppresses pc-3 solid tumor growth in a tumor xenograft mouse model. Int J Med Sci 11(6):593–601. https://doi.org/10.7150/ijms.8096
Lukasheva EV, Makletsova MG, Lukashev AN, Babayeva G, Arinbasarova AY, Medentsev AG (2020) Fungal enzyme l-lysine α-oxidase affects the amino acid metabolism in the brain and decreases the polyamine level. Pharmaceuticals 13:398. https://doi.org/10.3390/ph13110398
Lukasheva EV, Babayeva G, Zhdanov DD, Pokrovsky VS (2021) L-lysine α-oxidase: enzyme with anticancer properties. Pharmaceuticals. https://doi.org/10.3390/ph14111070
Machado ART, Aissa AF, Ribeiro DL, Costa TR, Ferreira RS Jr, Sampaio SV et al (2019) Cytotoxic, genotoxic, and oxidative stressinducing effect of an l-amino acid oxidase isolated from Bothrops jararacussu venom in a co-culture model of HepG2 and HUVEC cells. Int J Biol Macromol 127:425–432. https://doi.org/10.1016/j.ijbiomac.2019.01.059
Mondal K, Sharma A, Gupta MN (2003) Macroaffinity ligand-facilitated three phase partitioning for purification of glucoamylase and pullulanase using alginate. Protein Expres Purif 28:190–195
Moustafa IM, Foster S, Lyubimov AY, Vrielink A (2006) Crystal structure of LAAO from Calloselasma rhodostoma with an L-phenylalanine substrate: insights into structure and mechanism. J Mol Biol 364(5):991–1002
Mutaguchi Y, Ohmori T, Sakuraba H, Yoneda K, Doi K, Ohshima T (2011) Visible wavelength spectrophotometric assays of L-aspartate and D-aspartate using hyperthermophilic enzyme systems. Anal Biochem 409(1):1–6
Nakano M, Danowski TS (1966) Crystalline mammalian L-amino acid oxidase from rat kidney mitochondria. J Biol Chem 241(9):2075–2083
Nishizawa T, Aldrich CC, Sherman DH (2005) Molecular analysis of the rebeccamycin L-amino acid oxidase from Lechevalieria aerocolonigenes ATCC 39243. J Bacteriol 187(6):2084–2092. https://doi.org/10.1128/JB.187.6.2084-2092.2005
Nuutinen JT, Timonen S (2008) Identification of nitrogen mineralization enzymes, l-amino acid oxidases, from the ectomycorrhizal fungi Hebeloma spp. and Laccaria bicolor. Mycol Res 112(12):1453–1464. https://doi.org/10.1016/j.mycres.2008.06.023
Ozer B, Akardere E, Çelem EB, Onal S (2010) Three-phase partitioning as a rapid and efficient method for purification of invertase from tomato. Biochem Eng J 50(3):110–115
Pawelek PD, Cheah J, Coulombe R, Macheroux P, Ghisla S, Vrielink A (2000) The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site. EMBO J 19:4204–4215. https://doi.org/10.1093/emboj/19.16.4204
Pol MC, Deutsch HF, Visser L (1990) Purification of soluble enzymes from erythrocyte hemolysates by three phase partitioning. Int J Biochem 22:179–185
Ponnudurai G, Chung MC, Tan NH (1994) Purification and properties of the L-amino acid oxidase from Malayan pit viper (Calloselasmarhodostoma) venom. Arch Biochem Biophys 313(2):373–378
Puiffe ML, Lachaise I, Molinier-Frenkel V, Castellano F (2013) Antibacterial properties of the mammalian L-amino acid oxidase IL4I1. PLoS ONE 8(1):e54589
Rather GM, Gupta MN (2013) Three phase partitioning leads to subtle structural changes in proteins. Int J Biol Macromol 60:134–140
Rawdkuen S, Chaiwut P, Pintathong P, Benjakul S (2010) Three phase partitioning of protease from Calotropis procera latex. Biochem Eng J 50:145–149
Rey-Suarez P, Acosta C, Torres U, Saldarriaga-Córdoba M, Lomonte B, Núñez V (2018) MipLAAO, a new L-amino acid oxidase from the redtail coral snake. Peer J. 6:e4924. https://doi.org/10.7717/peerj.4924. (eCollection 2018)
Rodrigues RS, da Silva JF, França JB, Fonseca FP, Otaviano AR, Silva FH, Rodrigues VM (2009) Structural and functional properties of Bp-LAAO, a new L-amino acid oxidase isolated from Bothrops pauloensis snake venom. Biochimie 91(4):490–501
Sakurai Y, Takatsuka H, Yoshioka A, Matsui T, Suzuki M, Titani K et al (2001) Inhibition of human platelet aggregation by L-amino acid oxidase purified from Naja naja kaouthia venom. Toxicon 39:1827–1833. https://doi.org/10.1016/S0041-0101(01)00133-7
Sakurai Y, Shima M, Matsumoto T, Takatsuka H, Nishiya K, Kasuda S, Yoshioka A (2003) Anticoagulant activity of M-LAO, L-amino acid oxidase purified from Agkistrodon halys blomhoffii, through selective inhibition of factor IX. Biochimi Biophys Acta (BBA)-Proteins Proteom 1649(1):51–57
Salama WH, Ibrahim NM, El Hakim AE, Bassuiny RI, Mohamed MM, Mousa FM et al (2018) l-Amino acid oxidase from Cerastes vipera snake venom: Isolation, characterization and biological effects on bacteria and tumor cell lines. Toxicon 150:270–279. https://doi.org/10.1016/j.toxicon.2018.06.064
Samel M, Vija H, Ronnholm G, Siigur J, Kalkkinen N, Siigur E (2006) Isolation and characterization of an apoptotic and platelet aggregation inhibiting L-amino acid oxidase from Vipera berus berus (common viper) venom. Biochim Biophya Acta (BBA) Proteins Proteom 1764(4):707–714
Sant’Ana CD, Menaldo DL, Costa TR, Godoy H, Muller VD, Aquino VH, Soares AM (2008) RETRACTED: Antiviral and antiparasite properties of an l-amino acid oxidase from the Snake Bothrops jararaca: Cloning and identification of a complete cDNA sequence. Biochem Pharmacol 76:279–288
Shah S, Sharma A, Gupta MN (2004) Extraction of oil from Jatropha curcas seed kernels by enzyme assisted three phase partitioning. Ind Crop Prod 20:275–279
Sharma A, Gupta MN (2002) Macroaffinity Ligand-facilitated three phase partitioning for purification of xylanase. Biotechnol Bioeng 80(2):228–232
Sharma A, Sharma S, Gupta MN (2000) Purification of alkaline phosphatase from chicken intestine by three phase partitioning and use of phenyl-sepharose 6B in the batch mode. Biosep 9:155–161
Sharma A, Khare SK, Gupta MN (2002) Three phase partitioning for extraction of oil from soyabean. Bioresour Technol 85:327–329
Singh RK, Gourinath S, Sharma S, Roy I, Gupta MN, Betzel C, Srinivasan A, Singh TP (2001) Enhancement of enzyme activity through three phase partitioning: crystal structure of a modified serine proteinase at 1.5 A0. Protein Eng 14(5):307–313
Souza DHF, Eugenio LM, Fletcher JE, Jiang MS, Garratt RC, Oliva G et al (1999) Isolation and structural characterization of a cytotoxic L-amino acid oxidase from Agkistrodon contortrix laticinctus snake venom: preliminary crystallographic data. Arch Biochem Biophys 368(2):285–290. https://doi.org/10.1006/abbi.1999.1287
Stabeli RG, Marcussi S, Carlos GB, Pietro RCLR, Selistre-de-Araujo HS, Giglio JR et al (2004) Platelet aggregation and antibacterial effects of an L-amino acid oxidase purified from Bothrops alternatus snake venom. Bioorg Med Chem 12:2881–2886. https://doi.org/10.1016/j.bmc.2004.03.049
Sun Y, Nonobe E, Kobayashi Y, Kuraishi T, Sakai S, Aoki F, Yamamoto K (2002) Characterization and expression of L-amino acid oxidase of mouse milk. J Biol Chem 277(21):19080–19086
Szamos J, Kiss E (1995) Three phase partitioning of crude protein extracts. J Colloid Interf Sci 170:290–292
Tan NH, Saifuddin MN (1991) Substrate specificity of king cobra (Ophiophagus hannah) venom L-amino acid oxidase. Int J Biochem 23(3):323–327. https://doi.org/10.1038/srep42673
Tan KK, Ler SG, Gunaratne J, Bay BH, Ponnampalam G (2017) In vitro cytotoxicity of L-amino acid oxidase from the venom of Crotalus mitchellii pyrrhus. Toxicon 139:20–30. https://doi.org/10.1016/j.toxicon.2017.09.012
Tan KK, Bay BH, Gopalakrishnakone P (2018) L-amino acid oxidase from snake venom and its anticancer potential. Toxicon 144:7–13. https://doi.org/10.1016/j.toxicon.2018.01.015
Teixeira TL, Oliveira Silva VA, da Cunha DB, Polettini FL, Thomaz CD, Pianca AA et al (2016) Isolation, characterization and screening of the in vitro cytotoxic activity of a novel L-amino acid oxidase (LAAOcdt) from Crotalus durissus terrificus venom on human cancer cell lines. Toxicon 119:203–217. https://doi.org/10.1016/j.toxicon.2016.06.009
Tonismagi K, Samel M, Trummal K, Ronnholm G, Sugiir J, Kalkkinen N et al (2006) L-amino acid oxidase from Vipera lebetina venom: Isolation, characterization, effects on platelets and bacteria. Toxicon 48:227–237. https://doi.org/10.1016/j.toxicon.2006.05.004
Torii S, Naito M, Tsuruo T (1997) Apoxin I, a novel apoptosis-inducing factor with L-amino acid oxidase activity purified from Western diamondback rattlesnake venom. J Biol Chem 272(14):9539–9542. https://doi.org/10.1074/jbc.272.14.9539
Toyama MH, de Toyama DO, Passero LFD, Laurenti MD, Corbett CE et al (2006) Isolation of a new L-amino acid oxidase from Crotalus durissus cascavella venom. Toxicon 47:47–57. https://doi.org/10.1016/j.toxicon.2005.09.008
Ueda M, Chang CC, Ohno M (1988) Purification and characterization of L-amino acid oxidase from the venom of Trimeresurus mucrosquamatus (Taiwan habu snake). Toxicon 26(8):695–706. https://doi.org/10.1016/0041-0101(88)90276-0. (PMID: 3188059)
Ullah A (2020) Structure-function studies and mechanism of action of snake venom L-amino acid oxidases. Front Pharmacol 11:110
Ullah A, Masood R, Spencer PJ, Murakami MT, Arni RK (2014) Crystallization and preliminary X-ray diffraction studies of an L-amino-acid oxidase from Lachesis muta venom. Acta Crystallogr F Struct Biol Commun 70(Pt 11):1556–1559. https://doi.org/10.1107/S2053230X14017877
Vallon O, Bulte L, Kuras R, Olive J, Wollman FA (1993) Extensive accumulation of an extracellular l-amino-acid oxidase during gametogenesis of Chlamydomonas reinhardtii. Eur J Biochem 215(2):351–360
Van Boven A, Tan PST, Konings WN (1988) Purification and characterization of a dipeptidase from Streptococcus cremoris Wg2. Appl Environ Microbiol 54(1):43–49
Vetal MD, Shirpurkar ND, Rathod VK (2014) Three phase partitioning coupled with ultrasound for the extraction of urosolic acid and oleanolic acid from Ocimum sanctum. Food Bioprod Process 92(4):402–408
Vidhate GS, Singhal RS (2013) Extraction of cocoa butter alternative from kokum (Garcinia indica) kernel by three phase partitioning. J Food Eng 177:464–466
Vinayagam R, Vytla RM (2015) Partitioning of thermostable glucoamylase in polyethylene glycol/salt aqueous two-phase system. Bioresour Bioprocess 2:25. https://doi.org/10.1186/s40643-015-0056-6
Walter H, Larsson C (1994) Partitioning procedures and techniques: cell, organelles and membranes. In: Walter H, Johansson G (eds) Aqueous two-phase systems, methods in enzymology. Academic Press, California, pp 42–63
Wiezel GA, Rustiguel JK, Morgenstern D, Zoccal KF, Faccioli LH, Nonato MC et al (2019) Insights into the structure, function and stability of bordonein-L, the first L-amino acid oxidase from Crotalus durissus terrificus snake venom. Biochimie 163:33–49. https://doi.org/10.1016/j.biochi.2019.05.009
Yang H, Johnson PM, Ko KC, Kamio M, Germann MW, Derby CD, Tai PC (2005) Cloning, characterization and expression of escapin, a broadly antimicrobial FAD-containing L-amino acid oxidase from ink of the sea hare Aplysia californica. J Exp Biol 208(18):3609–3622
Yang HH, Yang SL, Peng KC, Lo CT, Liu SY (2009) Induced proteome of Trichoderma harzianum by Botrytis cinerea. Mycol Res 113(Pt 9):924–932. https://doi.org/10.1016/j.mycres.2009.04.004
Yu Z, Qiao H (2012) Advances in non-snake venom L-amino acid oxidase. Appl Biochem Biotechnol 167(1):1–13
Zainal Abidin SA, Rajadurai P, Chowdhury MEH, Ahmad Rusmili MR, Othman I, Naidu R (2018) Cytotoxic, antiproliferative and apoptosisinducing activity of L-amino acid oxidase from Malaysian Calloselasma rhodostoma on Human Colon cancer cells. Basic Clin Pharmacol Toxicol 123(5):577–588. https://doi.org/10.1111/bcpt.13060
Zhang L, Wei LJ (2007) ACTX-8, a cytotoxic L-amino acid oxidase isolated from Agkistrodon acutus snake venom, induces apoptosis in Hela cervical cancer cells. Life Sci 80(13):1189–1197
Zhang YJ, Wang JH, Lee WH, Wang Q, Liu H, Zheng YT, Zhang Y (2003) Molecular characterization of Trimeresurus stejnegeri venom L-amino acid oxidase with potential anti-HIV activity. Biochem Biophys Res Commun 309(3):598–604
Zhang H, Teng M, Niu L, Wang Y, Wang Y, Liu Q et al (2004) Purification, partial characterization, crystallization and structural determination of AHP-LAAO, a novel L-amino-acid oxidase with cell apoptosis-inducing activity from Agkistrodon halys pallas venom. Acta Crystallogr Sect D60:974–977. https://doi.org/10.1107/S0907444904000046
Zun G, Kos J, Sabotic J (2017) Higher fungi are a rich source of L-amino acid oxidases. 3 Biotech. 7(3):230
Author Information
Department of Bioscience and Biotechnology, Banasthali Vidyapeeth, Tonk, India