Gc–Ms profiling of bioactive compounds in Satyrium nepalense D. Don: unlocking its potential for herbal medicine
*Article not assigned to an issue yet
Research Articles | Published: 13 November, 2025
First Page: 0
Last Page: 0
Views: 109
Keywords: GC–MS, n Satyriumn , Orchidaceae, Bioactive compounds, Methanolic extract
Abstract
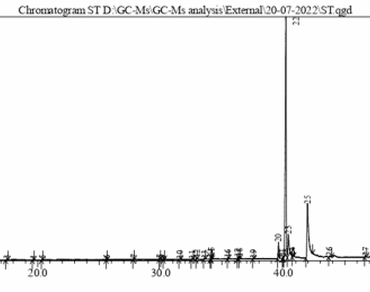
Satyrium nepalense D. Don belonging to the family Orchidaceae is a terrestrial herb, well known for its medicinal values since ancient times. In traditional medicine, the tubers of this species have been extensively used as aphrodisiac and to mitigate various fevers. The objective of the present study was to investigate the bioactive compounds of S. nepalense using its tubers with gas chromatography and mass spectroscopy analysis (GC–MS). During the present study, chromatogram analysis of the methanolic tuber extract showed the presence of thirty two compounds in which 2-Amino-3-(3,4-dimethoxyphenyl) propanoic acid (58.00%), Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester (21.64%), Ethanone, 2,2-dimethoxy-1,2-diphenyl- (8.41%), 9,19-Cyclolanost-24-en-3-ol, (3β)- (1.35%) were found to be prime compounds with known bioactivities such as antibacterial, antmicrobial, antiinflammatory, and antioxidant. The current study confirmed that the tubers of S. nepalense contain significant natural chemical compounds, validating its traditional use in various pharmacological activities.
References
Ahmad S, Alrouji M, Alhajlah S, Alomeir O, Pandey RP, Ashraf MS, Ahmad S, Khan S (2023) Secondary metabolite profiling, antioxidant, antidiabetic and neuroprotective activity of Cestrum nocturnum (night scented-jasmine): use of in vitro and in silico approach in determining the potential bioactive compound. Plants 12(6):1206. https://doi.org/10.3390/plants12061206.
Anneken D, Both S, Christoph R, Fieg G, Steinberner U, Westfechtel A (2006) Fatty acids. Ullmann’s Encycl Ind Chem. Weinheim Wiley-VCH. https://doi.org/10.1002/14356007.a10_245.pu.
Aparna V, Dileep KV, Mandal PK et al (2012) Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug des 80(3):434–439. https://doi.org/10.1111/j.1747-0285.2012.01418.x.
Beyer WEP, Palache AM, Reperant LA, Boulfich M, Osterhaus A (2020) Association between vaccine adjuvant effect and pre-seasonal immunity. Systematic review and meta-analysis of randomised immunogenicity trials comparing squalene-adjuvanted and aqueous inactivated influenza vaccines. Vaccine 38:1614–1622. https://doi.org/10.1016/j.vaccine.2019.12.037.
Chen X, Stephan WG, Phillip, JC (2009) Satyrium. In: Flora of China; Science Press, London. pp 165–166.
Deva S, Naithani HB (1986) The Orchid Flora of North West Himalaya. Print and Media Associates, New Delhi, India.
Dhillon MK, Pathak P (2023) Asymbiotic seed germination in a medicinally important and near threatened terrestrial orchid, Crepdidium acuminatum (D.Don) Szlach. from NorthWestern Himalayas: a study in vitro. J Orchid Soc India 37:49–57.
Duke JA (1992) Database of biologically active phytochemicals and their activity. CRC Press, Boca Raton, Florida, USA.
Garaniya N, Bapodra A (2014) Ethno botanical and phytophrmacological potential of Abrus precatorius L.: a review. Asian Pac J Trop Biomed 4:S27–S34. https://doi.org/10.12980/APJTB.4.2014C1069.
Harada H, Yamashita U, Kurihara H et al (2002) Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res 22(5):2587–2590.
Ibrahim N, Fairus S, Zulfarina MS, Naina Mohamed I (2020) The efficacy of squalene in cardiovascular disease risk-a systematic review. Nutrients 12:414. https://doi.org/10.3390/nu12020414.
Jaryal P, Pathak P, Warghat AR (2025a) An efficient clonal propagation of a medicinally important and endangered Himalayan herb, Dactylorhiza hatagirea D.Don Soo using shoot meristem culture and genetic fidelity analysis. Plant Cell Tissue Organ Cult 160:20. https://doi.org/10.1007/s11240-024-02958-4.
Jaryal P, Pathak P, Jaiswal V, Warghat AR (2025b) Identification of an endangered and medicinally important Himalayan orchid, Dactylorhiza hatagirea D.Don Soo using DNA Barcodes and development of an efficient in vitro propagation protocol utilising embryo culture technique. In Vitro Cell Dev Bio—Plant. https://doi.org/10.1007/s11627-025-10556-y.
Johnson SD, Peter CI, Ellis AG, Boberg E, Botes C, Van Der Niet T (2011) Diverse pollination systems of the twin-spurred orchid genus Satyrium in African grasslands. Plant Syst Evol 292:95–103.
Kalaiarasan A, John SA (2011) Some bioactive constituents of GC-MS analysis of Bulbophyllum kaitense Rechib stem Eastern Ghats of India. Int J Pharma Biol Sci 2:156–160.
Kim SK, Karadeniz F (2012) Biological importance and applications of squalene and squalane. Adv Food Nutr Res 65:223–233. https://doi.org/10.1016/B978-0-12-416003-3.00014-7.
Kim DH, Park MH, Choi YJ, Chung KW, Park CH (2013) Molecular study of dietary heptadecane for the anti-inflammatory modulation of NF-kB in the aged kidney. PLoSONE 8(3):e59316. https://doi.org/10.1371/journal.pone.0059316.
Kirti PP, Mahant KC (2023) Asymbiotic seed germination and seedling development in a commercially important and endemic orchid of Western Ghats, Aerides crispa Lindl.- a study in vitro. J Orchid Soc India 37:141–149.
Kumar P, Lomash V, Jatav PC, Kumar A, Pant SC (2016) Prenatal developmental toxicity study of n-heneicosane in Wistar rats. Toxicol Ind Health 32(1):118–125.
Kumar RS, Anburaj G, Subramanian A, Vasantha S, Selvam AP (2019) Preliminary phytochemical investigation, antimicrobial activity and GC-MS analysis of leaf extract of Capparis zeylanica Linn. J Pharm Phytochem 8:1399–1405.
Kumar A, Kaur S, Dhiman S, Singh PP, Thakur S, Sharma U, Kumar S, Kaur S (2021) 1,2-benzenedicarboxylic acid, bis (2-methyl propyl) ester isolated from Onosma bracteata Wall. inhibits MG-63 cells proliferation via Akt-p53-cyclin pathway. Res Square https://doi.org/10.21203/rs.3.rs-182390/v1.
Kumari A (2022) Studies on in vitro propagation, conservation and phytochemical analysis of some medicinally important orchids of Himachal Pradesh. PhD Thesis. Panjab University, Chandigarh.
Kumari A, Pathak P (2021) De novo plantlet regeneration from leaf explants of Rhynchostylis retusa (L.) Blume: A study in vitro. J Orchid Soc India 35:47–53.
Kumari A, Pathak P (2025) Phytochemical analysis of bioactive compounds in Calanthe tricarinata Lindl. pseudobulbs extract (orchidaceae) by gcms method. World J Pharm Pharm Sci 14(3):760–774.
Mahendran G, Bai VN (2009) Mass propagation of Satyrium nepalense D.Don.–a medicinal orchid via seed culture. Sci Hortic 119(2):203–207. https://doi.org/10.1016/j.scienta.2008.07.029.
Majumder S, Ghosh A, Chakraborty S, Bhattacharya M (2020) GC-MS analysis reveals Dendrobium candidum is a mosquito-attractant orchid with mosquitocidal compounds. Int J Mosquito Res 7(6):09–12.
Mendki MJ, Ganesan K, Shri P, Suryanarayana MV, Malhotra RC, Rao KM et al (2000) Heneicosane: an oviposition-attractant pheromone of larval origin in Aedes aegypti mosquito. Curr Sci 78(11):1295–1296.
Nasr ZS, El-Shershaby HM, Sallam KM, El-Din NN, El-Ghany IBY, Sidkey NM (2022) Evaluation of antimicrobial potential of tetradecane extracted from Pediococcus acidilactici DSM: 20284–CM isolated from curd milk. Egypt J Chem 65(3):705–713.
Noorjahan S, Rahamtulla M, Khasim SM (2023) Phytochemical profiling and GC-MS analysis of leaf eextracts of Dendrobiumanceps Sw. (Orchidaceae). J Med Pharmaceut Allied Sci 12(6):6230–6240.
Pathak P, Bhattacharya A, Vij SP, Mahant KC, Dhillon MK, Piri H (2010) An update on the medicinal orchids of Himachal Pradesh with brief notes on their habit, distribution, and flowering period. J Non Timber Forest Products 17(3):365–372.
Pathak P, Sunita KA, Thakur B, Vasundhra M (2022) Regeneration competence of an endangered orchid, Vanda cristata Wall. ex Lindl. using leaf explants: a study in vitro. S Afr J Bot 151:1018–1024.
Pathak P, Kumari A, Chandler B, Zettler LW (2023) In vitro propagation and phytochemical analysis of Vanda cristata Wall. ex Lindl.: an endangered medicinal orchid of biopharmaceutical importance. S Afr J Bot 153:109–123. https://doi.org/10.1016/j.sajb.2022.11.023.
Roy RN, Laskar S, Sen SK (2006) Dibutyl phthalate, the bioactive compound produced by Streptomyces albidoflavus 321.2. Microbiol Res 161(2):121–126.
Saxena S, Rao P (2018) GC-MS screening of bioactive constituents and antioxidant profiling in an invasive weed, Malvastrum coromandelianum (L.) Garcke. Pharma Innov 7:738–746.
Shaaban MT, Ghaly MF, Fahmi SM (2021) Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J Basic Microbiol 61(6):557–568. https://doi.org/10.1002/jobm.202100061.
Sharma S, Pathak P (2024) Elucidating the chemical composition and antioxidant activity of a therapeutically important and endangered orchid, Aerides multiflora Roxb. from northwestern Himalayas. World J Pharm Pharm Sci 13(10):742–765.
Singh SK, Agarwala DK, Jalal JS, Dash SS, Mao AA, Singh P (2019) Orchids of India: A Pictorial Guide. Botanical Survey of India, Kolkata, India.
Sudha T, Chidambarampillai S, Mohan VR (2013) GC-MS analysis of bioactive components of aerial parts of Fluggea leucopyrus Willd. (Euphorbiaceae). J Appl Pharm Sci 3:126–130.
Sunita PP, Mahant KC (2021) Green pod culture of an endangered and medicinally important orchid, Vanda cristata Wall. ex Lindl. from Himachal Pradesh. J Orchid Soc India 35:25–33.
Thakur B, Pathak P (2021) Application of organic additives for the enhancement of seed germination and seeding development in an endangered and medicinal orchid, Rhynchostylis retusa (L.) Blume through asymbiotic culture. J Orchid Soc India 35:99–107.
Thomas TP, Aneesh DG, Thomas RA (2013) GC-MS analysis of phytochemical compounds present in the rhizomes of Nervilia aragoana Gaud. Asian J Pharm Clin Res 6:68–74.
Usharani (2023) Anti-bacterial, anti-inflammatory activity and bio active compounds analysis of Curvularia lunata an endophytic fungi isolated from Justicia tranquebariensis-using GCMS. Int J Sci Res 12(4):1423–1427.
Vanitha V, Vijayakumar S, Nilavukkarasi M, Punitha VN, Vidhya E, Praseetha PK (2020) Heneicosane—a novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind Crop Prod 154:112748. https://doi.org/10.1016/j.indcrop.2020.112748.
Vasundhra, Pathak P, Anuprabha (2021) In vitro asymbiotic seed germination and regeneration competence of leaf explants in Satyrium nepalense D. Don, a medicinally important, and an endangered terrestrial orchid of Kasauli Hills, Himachal Pradesh (NorthWestern Himalayas). J Orchid Soc India. 35:73–82.
Verma S, Pathak P (2021) Effective use of synthetic seed technology in the regeneration of Cymbidium aloifolium using protocorm like bodies. Curr Sci 120(3):570–572.
Vij SP, Verma J, Kumar CS (2013) Orchids of Himachal Pradesh. Bishen Singh Mahendra Pal Singh, Dehra Dun, India.
Vinuchakkaravarthy T, Kumaravel KP, Ravichandran S, Velmurugan D (2011) Active compound from the leaves of Vitex negundo L. shows anti-inflammatory activity with evidence of inhibition for secretory phospholipase A(2) through molecular docking. Bioinformation 7(4):199–206. https://doi.org/10.6026/97320630007199.
Author Information
Orchid Laboratory, Department of Botany, Panjab University, Chandigarh, India