Genetic variability: physiological characteristics, pathogenicity and molecular diversity of Fusarium oxysporum f. sp. cumini infecting Cumin cyminum L. in India
Research Articles | Published: 05 March, 2020
First Page: 265
Last Page: 276
Views: 3883
Keywords: Cuminum cyminum L., Fusarium oxysporum f. sp. cumini (Foc), Genetic variability, Polymorphism, Pathogenicity, Species specificity
Abstract
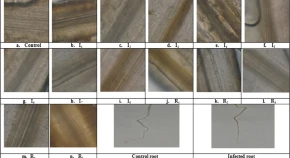
The fungal pathogen, Fusarium oxysporum f. sp. cumini (Foc) (soil born organism) is a severe menace to cumin cultivation in India and leads up to 80% of yield loss depending on disease severity. The present study was aimed to evaluate the diversities and similarities between isolates originating from different agro-climatic environments and to analyze pathogenicity in cumin plants. This study investigated a total of thirteen isolates of Fusarium oxysporum f. sp. cumini collected from different areas of Gujarat and Rajasthan. These isolates were screened and differentiated based on morphological characteristics such as colony character, color, pigmentation, and sporulation. Mycelial growth pattern showed discrimination among all isolates. The infestation rate of fungi on plants was diverse when analyzed with total proteins and activity of defense-related enzymes such as peroxidase, polyphenol oxidase, and catalase. They varied in pathogenicity when tested on var. GC-2 (susceptible variety). Junagadh isolate (I4) was found to be highly virulent amongst all. Genetic variability among the isolates was assessed using RAPD markers, a total of 693 loci were produced, out of which 651 were polymorphic with 93.99% average polymorphism. The species specificity was marked using internal transcribed spacer (ITS) primers with product amplification of approx. 437.24 bp in all the isolates. Overall, the results suggested that a high genetic variability and heterogeneity in Fusarium isolates call for the urge of gene identification from resistant cultivar and employ them in developing resistance breeding program combating the Fusarium oxysporum in cumin.
References
- Abd-Elsalam KA, Asran-Amal A, Schnieder F, Migheli Q, Verreet JA (2006) Molecular detection of Fusarium oxysporum f. sp. vasinfectum in cotton roots by PCR and real-time PCR assay. J Plant Dis Prot 113:14–19
- Agrios GN (1994) Plant pathology. Harcourt Academic Press, New York
- Arif M, Pani DR, Zaidi NW, Singh US (2011) PCR-based identification and characterization of Fusarium sp. associated with mango malformation. Biotechnol Res Int 141649:1–6
- Arnnok P, Ruangviriyachai C, Mahachai R, Techawongstien S, Chanthai S (2010) Optimization and determination of polyphenol oxidase and peroxidase activities in hot pepper (Capsicum annuum L.) pericarp. Int Food Res J 17:385–392
- Ascensao D, Dubey IA (2000) Panama disease: cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. sp. cubense race four. Phytopathology 90:1173
- Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, Ausubel FM, Paul Bolwell G (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47(6):851–863
- Boenisch MJ, Schäfer W (2011) Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol 11:110
- Bowden RL, Leslie JF (1992) Nitrate non-utilizing mutants of Gibberellazeae (Fusarium graminearum) and their use in determining vegetative compatibility. Exp Mycol 16:308–315
- Dange SRS (1995) Diseases of cumin (Cuminum cyminum L.) and their management. J Spices Aromat Crops 4(1):57–60
- Deshawal RK, Kumari N (2012) Regional variation in genetic structure and pathogenecity of Fusariumoxysporum f. sp. cumini isolated from Cuminum cyminum L. Asian J Biol Sci 5:30–38
- Divakar DEV, Anandraj M (2013) Cumin, fennel, and fenugreek. Soils, plant growth, and crop production. Encycl Life Supp Syst 1–10
- Dsouza R (2018) Jeera seasonal report. N.B. Commodities Research. https://www.nirmalbang.com/Upload/Jeera%20Seasonal%20Report%202018
- El-Fadly GB, El-Kazzaz MK, Hassan MAA, El-Kot GAN (2008) Identification of some Fusarium spp. using RAPD-PCR technique. Egypt J Phytopathol 36(1–2):71–80
- El-Khallal SM (2007) Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (Arbuscular mycorhizza) and/or hormonal elicitors (Jasmonic acid and salicylic acid): 2-changes in the antioxidant enzymes, phenolic compounds and pathogen related proteins. Aust J Basic Appl Sci 1:717
- Gordon TR, Okamoto D (1992) Variation within and between populations of Fusarium oxysporum based on vegetative compatibility and mitochondrial DNA. Can J Bot 70:1211–1217
- Jaccard P (1908) Nouvellesrecherchessur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
- Kanani P, Shukla YM, Modi AR, Subhash N, Kumar S (2018) Standardization of an efficient protocol for isolation of RNA from Cuminum cyminum. J King Saud Univ. https://doi.org/10.1016/j.jksus.2018.12.008
- Kawahara Y, Oono Y, Kanamori H, Matsumoto T, Itoh T, Minami E (2012) Simultaneous RNA-Seq analysis of a mixed transcriptome of rice and blast fungus interaction. PLoS ONE 7(11):e49423
- Khalequzzaman KM (2016) Effect of fungicides in controlling alternaria blight of cumin. Asian J Appl Sci Eng 5:7–14
- Kistler HC (2001) Evolution of host specificity in Fusarium oxysporum (eds Summerell). APS Press, St Paul, pp 70–82
- Kuvalekar A, Redkar A, Gandhe K, Harsulkar A (2011) Peroxidase and polyphenol oxidase activities in compatible host: pathogen interaction in Jasminum officinale and Uromyces hobsoni: Insights into susceptibility of host. N Z J Bot 49(3):351–359
- Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell Publishing, Iowa
- Levesque CA, Rahe JE, Eaves DM (1987) Effects of glyphosate on Fusarium spp. and its influence on root colonization of weeds, propagules density in the soil, and crop emergence. Can J Microbiol 33:354–360
- Lindahl BD, Henrik Nilsson R, Tedersoo L, Abarenkov K, Carlsen T, Kjøller R, Kõljalg U, Pennanen T, Rosendahl S, Stenlid J, Kauserud H (2013) Fungal community analysis by high-throughput sequencing of amplified markers - a user’s guide. New Phytol 199(1):288–299
- Lodha S, Mawar R (2014) Cumin wilt management: a review. J Spices Arom Crops 23(2):145–155
- Manikandan R, Subramanian H, Gandhi K, Thiruvengadam R (2018) Comparative proteomic analysis of different isolates of Fusarium oxysporum f sp lycopersici to exploit the differentially expressed proteins responsible for virulence on tomato plants. Front Microbiol 9:420
- McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Ann Rev Phytopathol 40:349–379
- Mehta S, Gaur VK, Sharma RA, Prasad J (2012) Assessment of genetic variability among Fusarium oxysporum f. sp. cumini isolates based on pathogenicity and RAPD markers. Indian Phytopathol 65:76–79
- Mohammadi A, Mofrad NN (2009) Genetic diversity in population of Fusarium solani from cumin in Iran. J Plant Prot Res 49(3):283–286
- Nawade BD, Jadeja KB, Talaviya JR, Vyas UM (2014) Comparative analysis of Fusarium oxysporum f. sp. cumini isolates using RAPD marker and cultural characteristics. Trends Biosci 7(17):2475–2478
- Nelson PE, Toussoun TA, Cook RJ (1981) Fusarium: diseases, biology, and taxonomy. Pennsylvania State University, State College
- Nelson BD, Hansen JM, Windels CE, Helms TC (1997) Reaction of soybean cultivars to isolates of Fusarium solani from Red River valley. Plant Dis 81:664–668
- Ozer G, Bayraktar H (2015) Intraspecific variation within Fusarium oxysporum f. sp. cumini from Cuminumcyminum in Turkey. Int J Agric Biol 17:375–380
- Percival GC (2001) Induction of systemic acquired disease resistance in plants: potential implications for disease management in urban forestry. J Arboric 27:181–192
- Pine L, Hoffman PS, Malcolm GB, Benson RF, Keen MG (1984) Determination of catalase, peroxidase, and superoxide dismutase within the genus legionella. J Clin Microbiol 20(3):421–429
- Saravanan T, Bhaskaran R, Muthusamy M (2004) Pseudomonas flurescens induced enzymological changes in banana roots against Fusarium wilt disease. Plant Pathol 3(2):72–80
- Sharma P, Samkumar A, Rao M, Singh VV, Prasad L (2018) Genetic diversity studies based on morphological variability, pathogenicity and molecular phylogeny of the Sclerotinia, sclerotiorum population from Indian Mustard (Brassica juncea). Front Microbiol 9:1169
- Sokal RR, Rohlf JF (1994) Biometry: the principles and practice of statistics in biological research, 4th edn. W.H. Freeman and Co., New York. https://doi.org/10.2307/2343822
- Song F, Goodman RM (2001) Molecular biology of disease resistance in rice. Physiol Mol Plant Pathol 59:111
- Tawfik AA, Allam ADA (2004) Improving cumin production under soil infestation with Fusarium wilt pathogen: I-screening of biocontrol agents. Ass Uni Bull Environ Res 7(2):35–44
- Wilson K, Walker J (2000) Practical biochemistry: principles and techniques. Cambridge University Press, Cambridge
- Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Ann Rev Phytopathol 26(1):379–407
- Zheng SJ, Fernando A, Bastidas G, Li X, Zeng L, Bai B, Xu S, Yin K, Li H, Fu G, Yu Y, Li Yang, Nguyen HC, Douangboupha B, Khaing AA, Drenth A, Seidl MF, Meijer HJG, KemaH J (2018) New geographical insights of the latest expansion of Fusarium oxysporum f. sp. cubense tropical race 4 into the Greater Mekong subregion. Front Plant Sci 9:457
Author Information
Department of Agricultural Biotechnology, Anand Agricultural University, Anand, India