Host seaweed extracts effect on epiphytic Acrochaetium sp growth and its phycobilipigments content and biological activity
Research Articles | Published: 27 March, 2024
First Page: 985
Last Page: 993
Views: 2890
Keywords: Epiphytic Acrochaetium sp., Seaweed-host nutrient, Phycobilipigments, Anti-oxidant activity, Anti-hyaluronidase
Abstract
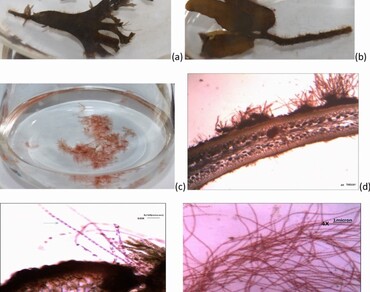
The association of microscopic epiphytic algae with marine macroalgae (seaweeds) hosts is common. One such epiphyte is Acrochaetium species and its growth under the influence of its host nutrients and bioprospecting is not investigated. In this work, the nutritional influence of host seaweed extracts red Gracilaria corticata, green Ulva fasciata, and brown Sargassum wightii, on the growth of Acrochaetium sp. CUKPSAK006 along with its phycobilipigments (PBPs) content and its biological activities was studied. The optimized laboratory conditions for the growth of Acrochaetium sp. found pH 7.6 and salinity 20‰ in MP1 medium. High growth is obtained in an MP1 medium supplemented with extracts of G. corticata and S. wightii but is insignificant compared to the control suggesting a facultative nutritional preference. However, PBPs possessing high anti-oxidant properties was found in the Acrochaetium sp. grown in the extracts of G. corticata and U. fasciata whereas significantly high hyaluronidase-inhibitory activity was observed in PBPs of Acrochaetium grown in the G. corticata extract. This study concludes that epiphytic microscopic Acrochaetium is a potential cultivable species for phycobilin pigment production either by defined medium or using seaweed extract supplement, considering the food and cosmetic values because of its good biological properties (anti-oxidant and anti-hyaluronidase).
References
Aráoz R, Lebert M, Häder DP (1998) Electrophoretic applications of phycobiliproteins. Electrophoresis 19:215–219. https://doi.org/10.1002/elps.1150190213
Arunkumar K, Raj R, Raja R, Carvalho IS (2021) Brown seaweeds as a source of anti-hyaluronidase compounds. S Afr J Bot 139:470–477. https://doi.org/10.1016/j.sajb.2021.03.036
Bhalodia N, Nariya P, Shukla V, Acharya R (2013) In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. AYU (An International Quarterly Journal of Research in Ayurveda) 34:209. https://doi.org/10.4103/0974-8520.119684
Clayden SL, Saunders GW (2014) A study of two Acrochaetium complexes in Canada with distinction of Rhododrewia gen. nov. (Acrochaetiales, Rhodophyta). Phycologia 53:221–232. https://doi.org/10.2216/13-224.1
Diez García YL, Jover Capote A, Suárez Alfonso AM, Gómez Luna LM, Fujii MT (2013) Distribution of epiphytic macroalgae on the thalli of their hosts in Cuba. Acta Bot Brasil 27:815–826. https://doi.org/10.1590/S0102-33062013000400022
Ferreira LB, Barufi JB, Plastino EM (2006) . 2: 187–192.
Francavilla M, Franchi M, Monteleone M, Caroppo C (2013) The red seaweed gracilaria gracilis as a multi products source. Mar Drugs 11:3754–3776. https://doi.org/10.3390/md11103754
Fujitani N, Sakaki S, Yamaguchi Y, Takenaka H (2001) Inhibitory effects of microalgae on the activation of hyaluronidase. J Appl Phycol 13:489–492
Glazer AN (1994) Phycobiliproteins - a family of valuable, widely used fluorophores. J Appl Phycol 6:105–112. https://doi.org/10.1007/BF02186064
Kedare SB, Singh RP (2011) Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 48:412–422. https://doi.org/10.1007/s13197-011-0251-1
Leonardi PI, Miravalles AB, Faugeron S, Flores V, Beltrán J, Correa JA (2006) Diversity, phenomenology and epidemiology of epiphytism in farmed Gracilaria chilensis (Rhodophyta) in northern Chile. Eur J Phycol 41:247–257. https://doi.org/10.1080/09670260600645659
Mittal R, Sharma R, Raghavarao KSMS (2019) Aqueous two-phase extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum. Biores Technol 280:277–286. https://doi.org/10.1016/j.biortech.2019.02.044
Ratnasooriya WD, Abeysekera WPKM, Ratnasooriya CTD (2014) In vitro anti-hyaluronidase activity of Sri Lankan low grown orthodox orange pekoe grade black tea (Camellia sinensis L.). Asian Pac J Trop Biomed 4:959–963. https://doi.org/10.12980/APJTB.4.2014APJTB-2014-0462
Sfriso AA, Gallo M, Baldi F (2018) Phycoerythrin productivity and diversity from five red macroalgae. J Appl Phycol 30:2523–2531. https://doi.org/10.1007/s10811-018-1440-3
Sonani R (2016) Recent advances in production, purification and applications of phycobiliproteins. World J Biol Chem 7:100. https://doi.org/10.4331/wjbc.v7.i1.100
Stegenga H, Van Erp ND (1979) Morphological variation in the genus Acrochaetium (Rhodophyta, Nemaliales). Acta Bot Neerl 28:425–448. https://doi.org/10.1111/j.1438-8677.1979.tb01168.x
Stegenga H, Vroman M (1976) The morphology and life history of Acrochaetium densum Papenfuss (Drew) Papenfuss European Zeeland) Netherlands, occurring epiphyte Dictyota preferably Chaetomorpha morpha Polysiphonia elongata (Eastern Polysiphonia Scheldt ) (Grevelingen, gical va. Acta Bot Need 25(4):257–280
Sudhakar MP, Jagatheesan A, Perumal K, Arunkumar K (2015) Methods of phycobiliprotein extraction from gracilaria crassa and its applications in food colourants. Algal Res 8:115–120. https://doi.org/10.1016/j.algal.2015.01.011
Tripathi SN, Kapoor S, Shrivastava A (2007) Extraction and purification of an unusual phycoerythrin in a terrestrial desiccation tolerant cyanobacterium Lyngbya arboricola. J Appl Phycol 19:441–447. https://doi.org/10.1007/s10811-006-9151-6
Van Der Velde HH (1973) The Use of phycoerythrin absorption spectra in the classification of red algae. Acta Bot Neerl 22:92–99. https://doi.org/10.1111/j.1438-8677.1973.tb00817.x
Vander VH (1973) The use of in phycoetythrin the absorption of spectra Algae. Acta Bot Neerl 22:92–99
Wang L, Wang S, Fu X, Sun L (2015) Characteristics of an R-phycoerythrin with two γ subunits prepared from red macroalga Polysiphonia urceolata. PLoS ONE 10:1–15. https://doi.org/10.1371/journal.pone.0120333
White EB, Boney AD (1969) Experiments with some endophytic and endozoic Acrochaetium species. J Exp Mar Biol Ecol 3:246–274. https://doi.org/10.1016/0022-0981(69)90050-1
Woelkerling WJ (1983) The Audouinella (Acrochaetium-Rhodochorton) complex (Rhodophyta): present perspectives WM. Phycologia 22:59–92
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43:27–32. https://doi.org/10.1021/jf00049a007
Author Information
Seaweed Group, Phycoscience Laboratory, Department of Plant Science, Central University of Kerala, Kasaragod, India