Impact of silicon dioxide nanoparticles on growth, photosynthetic pigments, proline, activities of defense enzymes and some bacterial and fungal pathogens of tomato
Research Articles | Published: 31 July, 2021
First Page: 83
Last Page: 93
Views: 4262
Keywords: Disease index, Disease management, Scanning electron microscopy, SiO2 NPs, Solanum lycopersicum
Abstract
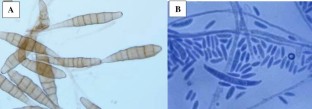
The effects of silicon dioxide nanoparticles (SiO2 NPs) in two concentrations (0.10 and 0.20 gL− 1) as foliar spray and seed priming was observed on plant growth attributes, chlorophyll, carotenoid, proline, activities of defense enzymes of tomato and on bacterial pathogens i.e. Pseudomonas syringae pv. tomato (Pst), Xanthomonas campestris pv. vesicatoria (Xcv), Pectobacterium carotovorum subsp. carotovorum (Pcc) and Ralstonia solanacearum (Rs), and fungal pathogens i.e. Fusarium oxysporum f. sp. lycopersici (Fol) and Alternaria solani (As) under in vitro and greenhouse conditions. Disease suppression and increase in plant growth was dependent on concentration of NPs and mode of application. Foliar spray was more effective than seed priming in increasing plant growth, chlorophyll, carotenoid, proline and activities of defense enzymes i.e. superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and phenylalanine ammonia lyase (PAL) in the presence and absence of pathogens. Foliar spray of 0.20 gL− 1SiO2 NPs caused highest increase in plant growth parameters, chlorophyll, carotenoid, proline and activities of defense enzymes in tomato plants and caused maximum reduction in disease indices. In vitro tests and scanning electron microscopy revealed antimicrobial effects of SiO2 NPs with varied adverse effects on pathogens under study. Plants subjected to foliar spray with 0.20 gL− 1SiO2 NPs had more plant growth attributes, chlorophyll, carotenoid, proline and defense enzymes against pathogens under study.
References
Aebi H (1984) Catalase in vitro. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 105. Academic Press, Florida, pp 114–121
Al-Samarrai AM (2012) Nanoparticles as alternative to pesticides in management plant diseases-a review. Int J Sci Res Publ 2(4):1–4
Al-Wakeel SA, Moubasher H, Gabr MMA, Madany MM (2013) Induced systemic resistance: an innovative control method to manage branched broomrape (Orobanche ramosa L.) in tomato. IUFS J Biol 72:9–21
Asada K (1992) Ascorbate peroxidase–a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85(2):235–241
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant soil 39(1):205–207
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some largeconsequences of minor changes in conditions. Anal Biochem 161:559–566
Capeletti LB, de Oliveira LF, Goncalves KDA, de Oliveira JFA, Saito A, Kobarg J, Cardoso MB (2014) Tailored silica–antibiotic nanoparticles: overcoming bacterial resistance with low cytotoxicity. Langmuir 30(25):7456–7464
Cecchini NM, Monteoliva MI, Alvarez ME (2011) Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiol 155:1947–1959
Christopher K, Bruno E (2003) Identification of bacterial species. In: O’Donnell MA (ed) Tested studies for laboratory teaching, vol 24, pp 103–130. Proceedings of the 24th workshop/conference of the Association for Biology Laboratory Education (ABLE)
Eichert T, Kurtz A, Steiner U, Goldbach HE (2008) Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol Plant 134(1):151–160
Elmer W, White JC (2018) The future of nanotechnology in plant pathology. Ann Rev Phytopathol 56:111–133
Fu PP, Xia Q, Hwang HM, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Analysis 22(1):64–75
Gul HT, Saeed S, Khan FZA, Manzoor SA (2014) Potential of nanotechnology in agriculture and crop protection: A. Appl Sci Bus Econ 1(2):23–28
Khan MR, Siddiqui ZA (2020) Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.). Sci Hortic 265:109211
Kim YH, Khan AL, Waqas M, Lee IJ (2017) Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Front Plant Sci 8:510
Laane HM (2018) The effects of foliar sprays with different silicon compounds. Plants 7(2):45
Ma JF, Yamaji N (2008) Functions and transport of silicon in plants. Cell Mol Life Sci 65:3049–3057. doi:https://doi.org/10.1007/s00018-008-7580-x
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Nesha R, Siddiqui ZA (2013) Interactions of Pectobacterium carotovorum pv. carotovorum, Xanthomonas campestris pv. carotae, and Meloidogyne javanica on the disease complex of carrot. Int J Veg Sci 19(4):403–411
Park HJ, Kim SH, Kim HJ, Choi SH (2006) A new compostion of nanosized silica-silver for control of various plant diseases. Plant Pathol J 22(3):295–302
Prochazhava D, Saivam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in miaze leaves. Plant Sci 161:756–771
Raliya R, Nair R, Chavalmane S, Wang WN, Biswas P (2015) Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7(12):1584–1594
Rastogi A, Tripathi DK, Yadav S, Chauhan DK, Živčák M, Ghorbanpour M, Brestic M (2019) Application of silicon nanoparticles in agriculture. 3 Biotech 9(3):90. https://doi.org/10.1007/s13205-019-1626-7
Riker AJ, Riker RS (1936) Introduction to research on plant diseases. Johns Swift Co., Louis, p 117
Sanoubar R, Barbanti L (2017) Fungal diseases on tomato plant under greenhouse condition. Eur J Biol Res 7(4):299–308
Schwab F, Zhai G, Kern M, Turner A, Schnoor JL, Wiesner MR (2016) Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–critical review. Nanotoxicology 10(3):257–278
Sharma PD (2001) Microbiology. Rastogi and Company, Meerut, India p 359
Shobha G, Moses V, Ananda S (2014) Biological synthesis of copper nanoparticles and its impact. Int J Pharm Sci Invent 3(8):6–28
Siddiqui MH, Al-Whaibi MH, Faisal M, Sahli A, A.A (2014) Nano‐silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem 33(11):2429–2437
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J Biol Sci 21(1):13–17
Siddiqui ZA, Hashmi A, Khan MR, Parveen A (2019) Management of bacteria Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, and fungi Rhizoctonia solani, Fusarium solani and Alternaria dauci with silicon dioxide nanoparticles on carrot. Int J Veg Sci 26(6):1–11
Smirnov NA, Kudryashov SI, Nastulyavichus AA, Rudenko AA, Saraeva IN, Tolordava ER, Zayarny DA (2018) Antibacterial properties of silicon nanoparticles. Laser Phys Lett 15(10):105602
Strout G, Russell SD, Pulsifer DP, Erten S, Lakhtakia A, Lee DW (2013) Silica nanoparticles aid in structural leaf coloration in the Malaysian tropical rainforest understorey herb Mapania caudata. Ann Bot 112(6):1141–1148
Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, Chauhan DK (2017) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–81
Uzu G, Sobanska S, Sarret G, Munoz M, Dumat C (2010) Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ Sci Technol 44(3):1036–1042
Valadkhan M, Mohammadi K, Nezhad MTK (2015) Effect of priming and foliar application of nanoparticles on agronomic traits of chickpea. Biol Forum 7:599
Xie Y, Li B, Zhang Q, Zhang C (2012) Effects of nano-silicon dioxide on photosynthetic fluorescence characteristics of Indocalamus barbatus McClure. J Nanjing Forest Univ (Natural Science Edition) 2:59–63
Yu C, Zeng LZ, Sheng K, Chen FX, Zhou T, Zheng XD, Yu T (2014) g-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem 159:29–37
Sneath PH, Sokal RR (1973) Numerical taxonomy. The principles and practice of numerical classification. W. H. Freeman and Company, San Francisco, USA, p 573
Author Information
Section of Plant Pathology and Nematology, Department of Botany, Aligarh Muslim University, Aligarh, India