In vitro antimicrobial and antioxidant activities of certain brackish water cyanobacteria from Chilika Lake, India
Research Articles | Published: 18 September, 2021
First Page: 38
Last Page: 50
Views: 4189
Keywords: Antimicrobial, Antioxidant, GC–MS, Phytochemicals, SOD
Abstract
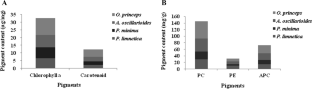
In the present investigation extracts of four species of cyanobacteria, (Pseudanabaena limnetica, Pseudanabaena minima, Anabaena oscillarioides and Oscillatoria princeps) using three solvents namely methanol, acetone and benzene were used to evaluate the pigments and phytochemical constituents, total phenolic, total flavonoid, antibacterial, antifungal and antioxidant activities. The test species possess distinctive amount of carotenoids and phycobiliproteins. The phytochemical screening indicated significant presence of alkaloids, flavonoids, saponins and resin. The antibacterial activity results indicated that, the methanol extract of O. princeps exhibited highest zone of inhibition i.e. 19 ± 1 mm and least MIC value (125 µg/ml) was obtained for Gram-negative pathogens than Gram-positive pathogens (250 µg/ml). The antifungal activity was more prominent in methanol extract of O. princeps against Candida albicans exhibiting higher zone of inhibition (20 ± 2 mm and MIC value 125 µg/ml). The antioxidant activity (DPPH. and ABTS assay) revealed highest activity in methanol extract of O. princeps (79.78% and 71.75%) and lowest was recorded in P. limnetica, (70.45% and 53.17%), while the standard (BHT) was 83.39% for DPPH and 75.04% for ABTS. Likewise, in DPPH assay, the IC50 for O. princeps and A. oscillarioides was (285.98 and 290.19 µg/ml) as compared to BHT (200.00 µg/ml) whereas for ABTS the IC50 was 338.29 and (339.15 µg/ml) against the standard (BHT) 274.07 µg/ml. O. princeps exhibited highest SOD activity as well as protein content (28.4 U/mg and 38.13 mg/g) and lowest was observed in P limnetica (11.8 U/mg and 10.03 mg/g). In GC–MS analysis a total of twelve bioactive compounds were identified in methanol extract of O. princeps. The Methyl-11-Octadecenoate with percentage composition of (28.23%) was the major compound followed by Methyl Hexadecanoate (26.14%). Hence, the present study revealed that cyanobacteria thriving in Chilika lake possess excellent antimicrobial and antioxidants potential and further exploration can open new horizon.
References
Afroz R, Tanvir EM, Islam MA, Alam F, Gan SH, Khalil MI et al (2014) Potential antioxidant and antibacterial properties of a popular jujube fruit: apple Kul (Zizyphus mauritiana). J Food Biochem 38(6):592–601
Ahamed AAP, Rasheed MU, Noorani KPM, Reehana N, Santhoshkumar S, Imran YMM, Alharbi NS, Arunachalam C, Alharbi SA, Akbarsha MA, Thajuddin N et al (2017) In vitro antibacterial activity of MGDG-palmitoyl from Oscillatoria acuminata NTAPC05 against extended-spectrum β-lactamase producers. J Antibiot 70(6):754–762
Anahas AMP, Muralitharan G (2015) Isolation and screening of heterocystous cyanobacterial strains for biodiesel production by evaluating the fuel properties from fatty acid methyl ester (FAME) profiles. Biores Technol 184:9–17
Anahas AMP, Muralitharan G (2018) Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energy Convers Manag 157:423–437
Ashwini P, Krishnamoorthy M (2011) Antioxidant activity of ethanolic extract of Cassia tora L. Int J Res Ayurveda Pharm 2(1):25–252
Babić O, Kovač D, Rašeta M, Šibul F, Svirčev Z, Simeunović J et al (2016) Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. J Appl Phycol 28(4):2333–2342
Chang CC, Yang MH, Wen HM, Chern JC et al (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10(3):178–182
Chauhan J, Kasture A (2014) Antimicrobial compounds of marine algae from Indian coast. Int J Curr Microbiol Appl Sci 7:526–532
Das K, Samanta L, Chainy GBN et al (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37:201–204
Desikachary TV (1959) Cyanophyta, vol 2. Indian Council of Agricultural Research, New Delhi, p 686
Dhanalakshmi M, Angayarkanni J (2013) Phytochemistry and antibacterial activity of Chlorosarcinopsis species. Int J Sci Technol Res 2:315–321
Elshouny WA, El-Sheekh MM, Sabae ShZ, Khalil MA, Badr HM et al (2017) Antimicrobial activity of spirulina platensis against aquatic bacterial isolates. J Microbiol Biotechnol Food Sci 6:1203–1208
Gunes S, Tamburaci S, Imamoglu E, Dalay MC et al (2015) Determination of superoxide dismutase activities in different cyanobacteria for scavenging of reactive oxygen species. J Biol Active Prod Nat 5(1):25–32
Hussain AI, Anwar F, Sherazi STH, Przybylski R (2008) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimumbasilicum) essential oils depends on seasonal variations”. Food Chem 108(3):986–995
Jena J, Subudhi E (2019) Microalgae: An untapped resource for natural antimicrobials. In: Sukla LB, Subudhi E, Pradhan D (eds) The role of microalgae in wastewater treatment. Springer, Singapore, pp 99–114
Jensen A (1978) Chlorophylls and carotenoids. In: Hellebust JA, Craigie JS (eds) Handbook of physiological methods: physiological and biochemical methods. Cambridge University Press, pp 59–70
Kannan RRR, Arumuga R, Anantharaman P et al (2010) In vitro antioxidant activities of ethanol extract from Enhalus acoroides. Asian Pac J Trop Med 3:898–901
Kosanić M, Ranković B, Vukojević J (2011) Antioxidant properties of some lichen species. J Food Sci Technol 48(5):584–590
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li HB, Cheng KW, Wong CC, Fan KW, Chen F, Jiang Y et al (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 102(3):771–776
Liu J, Wen XY, Zhang XQ, Pu HM, Kan J, Jin CH (2015) Extraction, characterization and in vitro antioxidant activity of polysaccharides from black soybean. Int J Biol Macromol 72:1182–1190
Machu L, Misurcova L, Vavra Ambrozova J, Orsavova J, Mlcek J, Sochor J, Jurikova T et al (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20(1):1118–1133
Madhumathi V, Deepa P, Jeyachandran S, Manoharan C, Vijayakumar S (2011) Antimicrobial activity of cyanobacteria isolated from freshwater lake. Int J Microbiol 2(3):213–216
Marrez DA, Sultan YY, Embaby MA (2017) Biological activity of the cyanobacteriumOscillatoriabrevisextracts as a source of nutraceutical and bio-preservative agents. Int J Pharmacol 13(8):1010–1019
Martínez-Frances E, Escudero-Onate C (2018) Cyanobacteria and microalgae in the production of valuable bioactive compounds. Microalgal Biotechnol 6:104–128. https://doi.org/10.5772/intechopen.74043
McDonald S, Prenzler PD, Antolovich M, Robards K (2001) Phenolic content and antioxidant activity of olive extracts. Food Chem 73(1):73–84
Mckinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Minkova KM, Toshkova RA, Gardeva EG, Tchorbadjieva MI, Ivanova NJ, Yossifova LS, Gigova LG et al (2011) Antitumor activity of B-phycoerythrin from Porphyridium cruentum. J Pharm Res 4(5):1480–1482
Miranda MS, Cintra RG, Barros SDM, Mancini-Filho J (1998) Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res 31(8):1075–1079
Ordonez AAL, Gomez JD, Vattuone MA (2006) Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem 97(3):452–458
Oroian M, Escriche I (2015) Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int 74:10–36
Pandey VD (2015) Cyanobacterial natural products as antimicrobial agents. Int J Curr Microbiol App Sci 4(1):310–317
Pan-utai W, Iamtham S (2019) Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem 82:189–198
Priyadarshani I, Rath B (2012) Commercial and industrial application of microalgae—a review. J Algal Biomass Util 3(4):89–100
Pumas C, Vacharapiyasophon P, Peerapornpisal Y, Leelapornpisid P, Boonchum W, Ishii M, Khanongnuch C et al (2011) Thermostablility of phycobiliproteins and antioxidant activity from four thermotolerant cyanobacteria. Phycol Res 59(3):166–174
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237
Sanja SD, Sheth NR, Patel NK, Patel D, Patel B et al (2009) Characterization and evaluation of antioxidant activity of Portulaca oleracea. Int J Pharm Pharm Sci 1(1):5–10
Scazzocchio F, Comets MF, Tomassini L, Palmery M et al (2001) Antibacterial activity of Hydrastiscanadensis extract and its major isolated alkaloids. Planta Med 67:561–563
Sethubathi GVB, Prabu VA (2010) Antibacterial activity of cyanobacterial species from adirampattinam coast, southeast coast of palk bay. Curr Res J Biol Sci 2(1):24–26
Shanab SM, Mostafa SS, Shalaby EA, Mahmoud GI et al (2017) Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. Asian Pac J Trop Biomed 2(8):608–615
Sharathchandra K, Rajashekhar M (2013) Antioxidant activity in the four species of cyanobacteria isolated from a sulfur spring in the Western Ghats of Karnataka. Int J Pharm Bio Sci 4(1):275–285
Siegelman H, Kycia JH (1978) Alga biliproteins. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, pp 72–78
Soltani N, Khavari-Nejad RA, Yazdi MT, Shokravi S, Fernández-Valiente E et al (2006) Variation of nitrogenase activity, photosynthesis and pigmentation of the cyanobacterium Fischerella ambigua strain FS18 under different irradiance and pH values. World J Microbiol Biotechnol 22(6):571
Sonani RR, Singh NK, Kumar J, Thakar D, Madamwar D et al (2014) Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochem 49(10):1757–1766
Srinivasan D, Nathan S, Suresh T, Lakshmana PP et al (2001) Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol 74:217–220
SushanthRai SV, Rajashekhar M (2015) Antioxidant potential of eight species of cyanobacteria isolated from Arabian Sea coast of Karnataka. J Chem Pharm Res 7(12):938–942
Swamy MK, Arumugam G, Kaur R, Ghasemzade A, Yusoff MM, Sinniah UR et al (2017) GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid-Based Complement Altern Med 2107:1–10
Takaichi S (2011) Carotenoids in algae: distributions, biosyntheses and functions. Mar Drugs 9(6):1101–1118
Author Information
Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University, Baripada, India