In vitro micropropagation and genetic fidelity studies using SCoT and ISSR primers in Annona reticulata L.: an important medicinal plant
Kudikala Hemalatha, Jogam Phanikanth, Sirikonda Abhiteja, Mood Kasim, Allini Venkateswar Rao
Research Articles | Published: 23 June, 2020
First Page: 446
Last Page: 457
Views: 4090
Keywords: Annona reticulata , Multiple shoot induction, Genetic fidelity, SCoT, ISSR
Abstract
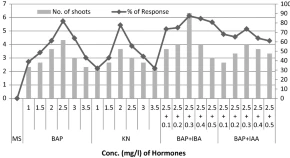
An efficient and reproducible in vitro plant regeneration protocol has been developed for Annona reticulata L., a medicinally important plant of family Annonaceae. This is the first report of an efficient in vitro micropropagation and genetic fidelity evaluation of Annona reticulata using nodal segments as explants. Nodal explants were cultured on Murashige and Skoog (MS) medium augmented with various concentrations of cytokinins alone and in combination with auxins. MS medium supplemented with 2.5 mg/l 6-benzylaminopurine (BAP) and 0.3 mg/l indole-3-butyricacid (IBA) combination has proven the best for optimum regeneration response (87.4%) and a maximum number of multiple shoots (6.33 ± 0.37). Rhyzogenesis occurred on half-strength MS medium supplemented with 4 mg/l IBA, resulted in the highest percentage of rooting (73%) and a maximum number of roots (4.65 ± 0.23) along with maximum root length (1.76 ± 0.08 cm). The well developed regenerates were shifted to the mixture of soil, sand and organic manure (2:1:1) in small plastic cups and acclimatized successfully. Genetic fidelity of the discussed etiquette was validated using two types of DNA fingerprinting techniques i.e. start codon targeted (SCoT), and inter simple sequence repeat (ISSR) analyses. Among the ten SCoT (SC) and ISSR primers used, an excellent amplification with scorable DNA bands was produced by SC9 and ISSR4 primers. The results showed that the regenerated plantlets are monomorphic and true-to-type with mother plant. This study provides important information about selection of suitable regeneration medium to improve multiple shoot induction and rooting of Annona reticulata. It can be used for commercial cultivation and for further genetic redevelopment investigation studies, also applied for large scale propagation of elite genotypes.
References
- Ahmed MR, Anis M, Alatar AA, Faisal M (2017) In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: a potential medicinal plant. Agrofor Syst 91(4):637–647
- Auer CA, Motyka V, Březinová A, Kamínek M (1999) Endogenous cytokinin accumulation and cytokinin oxidase activity during shoot organogenesis of Petunia hybrida. Physiol Plant 105(1):141–147
- Balakrishnan V, Latha MR, Ravindran KC, Robinson JP (2009) Clonal propagation of Morus alba L. through nodal and axillary bud explants. Bot Res Int 2(1):42–49
- Baskaran P, Jayabalan N (2008) Effect of growth regulators on rapid micropropagation and psoralen production in Psoralea corylifolia L. Acta Physiol Plant 30(3):345–351
- Batista D, Sousa MJ, Pais MS (1996) Plant regeneration from stem and petiole-derived callus of Humulus lupulus L. (hop) clone Bragança and var. Brewer’s Gold. In Vitro-Plant 32(1):37–41
- Bekheet SA, Gabr AM, Reda AA, El Bahr MK (2015) Micropropagation and assessment of genetic stability of In Vitro raised jojoba (Simmondsia chinensis Link.) plants using SCoT and ISSR markers. Plant Tissue Cult Biotechnol 25(2):165–179
- Benson EE (2000) In vitro plant recalcitrance: an introduction. In Vitro Cell Dev Biol Plant 36(3):141–148
- Bhalke RD, Chavan MJ (2011) Analgesic and CNS depressant activities of extracts of Annona reticulata L. bark. Phytopharmacology 1(5):160–165
- Chand S, Singh AK (2004) In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree Pterocarpus marsupium Roxb. In Vitro Cell Dev Biol Plant 40(5):464–466
- Chavan MJ, Kolhe DR, Wakte PS, Shinde DB (2012a) Analgesic and antiinflammatory activity of Kaur-16-en-19-oic acid from Annona reticulata L. Bark Phytother Res 6(2):273–276
- Chavan MJ, Wakte PS, Shinde DB (2012b) Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat Prod Res 26(16):1515–1518
- Collard BC, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27(1):86
- Doyle JJ, Doyle JL (1990) Isolation ofplant DNA from fresh tissue. Focus 12(13):39–40
- D'Souza S, Fernandes J, Kumar VM, D'Souza NG, Fernandes R (2019) Antiepileptic activity of ethanolic and aqeous leaves extract of Annona reticulata L. Res J Pharm Technol 12(1):241–244
- Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11(1):1–42
- Duval RA, Duret P, Lewin G, Peris E, Hocquemiller R (2005) Semisynthesis and biological activity of aminoacyl triesters of squamocin, an annonaceous acetogenin. Bioorg Med Chem 13(11):3773–3781
- Elayaraja D, Subramanyam K, Vasudevan V, Sathish S, Kasthurirengan S, Ganapathi A, Manickavasagam M (2019) Meta-topolin (mT) enhances the in vitro regeneration frequency of Sesamum indicum (L.). Biocatal Agric Biotechnol 21:101320
- Fang-Yong C, Ji-Hong L (2014) Germplasm genetic diversity of Myrica rubra in Zhejiang province studied using inter-primer binding site and start codon-targeted polymorphism markers. Sci Hortic 170:169–175
- Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol Plant 32(4):272–289
- George AP, Nissen RJ (1987) Propagation of Annona species: a review. Sci Hortic 33(1–2):75–85
- Gupta AK, Rai MK, Phulwaria M, Agarwal T, Shekhawat NS (2014) In vitro propagation, encapsulation, and genetic fidelity analysis of Terminalia arjuna: a cardioprotective medicinal tree. Appl Biochem Biotechnol 173(6):1481–1494
- Gurriaran MJ, Revilla MA, Tames RS (1999) Adventitious shoot regeneration in cultures of Humulus lupulus L. (hop) cvs. Brewers Gold and Nugget. Plant Cell Rep 18(12):1007–1011
- Hossain SN, Munshi MK, Islam MR, Hakim L, Hossain M (2003) In vitro propagation of plum (Zyziphus jujuba Lam.). Plant Tissue Cult 13(1):81–84
- Islam MR, Rahman SM, Ahmed M, Das PR, Tabibul M, Islam MH, Ahmad I, Rahmatullah M (2012) Antinociceptive activity studies with methanol extract of Annona reticulata L. (Annonaceae) and Carissa carandas L. (Apocynaceae) leaves in Swiss albino mice. Adv Nat Appl Sci 6(8):1313–1318
- Jamkhande PG, Wattamwar AS, Pekamwar SS, Chandak PG (2014) Antioxidant, antimicrobial activity and in silico PASS prediction of Annona reticulata Linn. root extract. Beni-Suef Univ J Basic Appl Sci 3(2):140–148
- Kirika MW, Kahia JW, Diby LN, Njagi EM, Dadjo C, Kouame C (2015) Micropropagation of an endangered medicinal and indigenous multipurpose tree species: Erythrina abyssinica. Hort Sci 50(5):738–743
- Konar S, Adhikari S, Karmakar J, Ray A, Bandyopadhyay TK (2019) Evaluation of subculture ages on organogenic response from root callus and SPAR based genetic fidelity assessment in the regenerants of Hibiscus sabdariffa L. Ind Crops Prod 135:321–329
- Kudikala H, Ellendula R, Nazrin S, Sirikonda A, Mood K, Allini VR (2018) Research article effect of pre-treatment methods on in vitro seed germination of bullock’s heart (Annona reticulata L.). Asian J Plant Sci 17:142–149
- Larkin PJ, Scowcroft WR (1981) Somaclonal variation a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60(4):197–214
- Letham DS, Palni LM (1983) The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol 34(1):163–197
- Modgil M, Mahajan K, Chakrabarti SK, Sharma DR, Sobti RC (2005) Molecular analysis of genetic stability in micropropagated apple rootstock MM106. Sci Hortic 104(2):151–160
- Mondal SK, Mondal NB, Mazumder UK (2007) In vitro cytotoxic and human recombinant caspase inhibitory effect of Annona reticulata leaves. Indian J Pharmacol 39(5):253
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
- Nasri A, Baklouti E, Romdhane AB, Maalej M, Schumacher HM, Drira N, Fki L (2019) Large-scale propagation of Myrobolan (Prunus cerasifera) in RITA® bioreactors and ISSR-based assessment of genetic conformity. Sci Hortic 245:144–153
- Nirmal SA, Gaikwad SB, Dhasade VV, Dhikale RS, Kotkar PV, Dighe SS (2010) Anthelmintic activity of Annona reticulata leaves. Res J Pharm Biol Chem Sci 1(1):115–118
- Parthiban E, Arokiyaraj C, Janarthanan S, Ramanibai R (2019a) Antioxidant and GC–MS analysis of Annona reticulata leaves extract against unsecure free radicals. SN Appl Sci 1(4):349
- Parthiban E, Manivannan N, Ramanibai R, Mathivanan N (2019b) Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol Rep 21:e00297
- Patel AK, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS (2014) In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f.: an endemic and endangered edible plant species of the Thar Desert. Sci Hortic 165:175–180
- Pati PK, Rath SP, Sharma M, Sood A, Ahuja PS (2006) In vitro propagation of rose—a review. Biotechnol Adv 24(1):94–114
- Patil SB, Chavan GM, Ghodke DS, Naikwade NS, Magdum CS (2009) Screening of some indigenous plants for their antipyretic activity. Res J Pharmacol Pharmacodyn 1(3):143–144
- Phulwaria M, Rai MK, Gupta AK, Ram K, Shekhawat NS (2012) An improved micropropagation of Terminalia bellirica from nodal explants of mature tree. Acta Physiol Plant 34(1):299–305
- Rahman SM, Rashedul MI, Rahman S, Mosaiab T, Ahmed R, Khatun F (2011) Antihyperglycemic studies with methanol extract of Annona reticulata L. (Annonaceae) and Carissa carandas L. (Apocynaceae) leaves in Swiss albino mice. Adv Nat Appl Sci 5(2):218–222
- Rahmani MS, Pijut PM, Shabanian N, Nasri M (2015) Genetic fidelity assessment of in vitro-regenerated plants of Albizia julibrissin using SCoT and IRAP fingerprinting. In Vitro Cell Dev Biol Plant 51(4):407–419
- Rai MK, Asthana P, Jaiswal VS, Jaiswal U (2010) Biotechnological advances in guava (Psidium guajava L.): recent developments and prospects for further research. Trees 24(1):1–2
- Rai MK, Phulwaria M, Gupta AK, Shekhawat NS, Jaiswal U (2012) Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tissue Organ Cult (PCTOC) 111(2):259–264
- Raji MR, Lotfi M, Tohidfar M, Zahedi B, Carra A, Abbate L, Carimi F (2018) Somatic embryogenesis of muskmelon (Cucumis melo L.) and genetic stability assessment of regenerants using flow cytometry and ISSR markers. Protoplasma 255(3):873–883
- Ramesh M, Umate P, Rao KV, Sadanandam A (2005) Micropropagation of Terminalia bellirica Roxb. A sericulture and medicinal plant. In Vitro Cell Dev Bio-Plant 41(3):320–323
- Rani V, Raina SN (2000) Genetic fidelity of organized meristem-derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant 36(5):319–330
- Rathore MS, Mastan SG, Yadav P, Bhatt VD, Shekhawat NS, Chikara J (2016) Shoot regeneration from leaf explants of Withania coagulans (Stocks) Dunal and genetic stability evaluation of regenerates with RAPD and ISSR markers. S Afr J Bot 102:12–17
- Rathore NS, Rai MK, Phulwaria M, Rathore N, Shekhawat NS (2014) Genetic stability in micropropagated Cleome gynandra revealed by SCoT analysis. Acta Physiol Plant 36(2):555–559
- Rohela GK, Jogam P, Bylla P, Reuben C (2019) Indirect regeneration and assessment of genetic fidelity of acclimated plantlets by SCoT, ISSR, and RAPD markers in Rauwolfia tetraphylla L.: an endangered medicinal plant. BioMed Res Int 2019:1–14
- Rohela GK, Jogam P, Shabnam AA, Shukla P, Abbagani S, Ghosh MK (2018) In vitro regeneration and assessment of genetic fidelity of acclimated plantlets by using ISSR markers in PPR-1 (Morus sp.): An economically important plant. Sci Hortic 241:313–321
- Rout GR, Senapati SK, Aparajeta S (2008) Micropropagation of Acacia chundra (Roxb.) DC. Hort Sci 35:22–26
- Sajeevan RS, Singh SJ, Nataraja KN, Shivanna MB (2011) An efficient in vitro protocol for multiple shoot induction in mulberry, Morus alba L variety V1. Int Res J Plant Sci 2(8):254–261
- Saker MM, Bekheet SA, Taha HS, Fahmy AS, Moursy HA (2000) Detection of somaclonal variations in tissue culture-derived date palm plants using isoenzyme analysis and RAPD fingerprints. Biol Plant 43(3):347–351
- Senadeera SS, Prasanna PH, Jayawardana NW, Gunasekara DC, Senadeera P, Chandrasekara A (2018) Antioxidant, physicochemical, microbiological, and sensory properties of probiotic yoghurt incorporated with various Annona species pulp. Heliyon 4(11):e00955
- Silva KD, Sirasa MS (2018) Antioxidant properties of selected fruit cultivars grown in Sri Lanka. Food Chem 238:203–208
- Singh JA, Kumar SV, Kadam VA (2012) Antiulcer activity of Annona reticulata leaves extract in rats. Int J Pharm Pharm Sci 4(1):412–414
- Singh SK, Rai MK, Asthana P, Sahoo L (2009) An improved micropropagation of Spilanthes acmella L. through transverse thin cell layer culture. Acta Physiol Plant 31(4):693–698
- Song JY, Sivanesan I, An CG, Jeong BR (2010) Adventitious shoot regeneration from leaf explants of miniature paprika (Capsicum annuum)‘Hivita Red’and ‘Hivita Yellow’. Afr J Biotechnol 9(19):2768–2773
- Sriskandarajah S, Prinsen E, Motyka V, Dobrev PI, Serek M (2006) Regenerative capacity of cacti Schlumbergera and Rhipsalidopsis in relation to endogenous phytohormones, cytokinin oxidase/dehydrogenase, and peroxidase activities. J Plant Growth Regul 25(1):79–88
- Sudhersan C, Hussain J (2003) In vitro clonal propagation of a multipurpose tree, Ziziphus spina-christi (L.) Desf. Turk J Bot 27(3):167–172
- Suresh HM, Shivakumar B, Hemalatha K, Heroor SS, Hugar DS, Rao KS (2011) In vitro antiproliferativeactivity of Annona reticulata roots on human cancer cell lines. Pharmacogn Res 3(1):9
- Tewary PK, Raghunath MK, Venkateshwarulu M, Sarkar A (1996) Genotypic difference in response to in vitro shoot development of mulberry (Morus spp.). Indian J Sericult 35(2):104–106
- Thakur J, Dwivedi MD, Sourabh P, Uniyal PL, Pandey AK (2016) Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle—an endemic and endangered medicinal plant. PLoS ONE 11(7):e0159050
- Thiyagarajan M, Venkatachalam P (2012) Large scale in vitro propagation of Stevia rebaudiana (bert) for commercial application: Pharmaceutically important and antidiabetic medicinal herb. Ind Crops Prod 37(1):111–117
Author Information
Department of Biotechnology, Kakatiya University, Warangal, India