In vitro physiological and biochemical responses underlying salt tolerance of five turfgrass species
*Article not assigned to an issue yet
Wei Zhen-Peng, Xiong Yu-Ping, da Silva Jaime A. Teixeira, Li Jian-Rong, Liu Jun-Yu, Li Yuan, Bian Zhan, Zhang Xin-Hua, Ma Guo-Hua, Xiong Yu-Ping
Research Articles | Published: 03 September, 2025
First Page: 0
Last Page: 0
Views: 4
Keywords: Halophytes, Lawn plants, Physiological responses, Tissue culture, Salt tolerance
Abstract
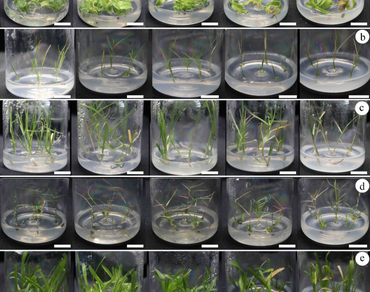
Turfgrasses growing on tropical island reefs have developed morphological, structural, physiological and ecological strategies that confer them with tolerance to salt, high temperatures and drought. They are thus able to adaptively survive in extreme environments with high salt concentrations, high temperatures, strongly alkaline soil, and high light intensities. Research on tropical island turfgrasses advance knowledge on island ecological restoration, salt tolerance, and resource development. This study focused on five turfgrass species that are used as lawn turfgrass in southern China: Lepturus repens (G. Forst.) R. Br., Thuarea involuta (Forst.) R. Br, Zoysia matrella (L.) Merr, Cynodon dactylon (L.) Persoon and Axonopus compressus (Sw.) P. Beauv. For all species, using an in vitro tissue culture system based on Murashige and Skoog (MS) basal medium for shoot proliferation and growth, the physiological responses to different NaCl concentrations were assessed, and NaCl tolerance was evaluated. Based on physiological or biochemical parameters, salt tolerance of the species was A. compressus (0.06) > Z. matrella (0.09) > C. dactylon (0.14) > L. repens (0.42) > T. involuta (0.67) at 50 mM NaCl; Z. matrella (0.04) > A. compressus (0.25) > C. dactylon (0.35) > L. repens (0.46) > T. involuta (0.67) at 100 mM; C. dactylon (0.30) > L. repens (0.37) > A. compressus (0.48) > Z. matrella (0.60) > T. involuta (0.95) at 200 mM NaCl; C. dactylon (0.33) > L. repens (0.46) > Z. matrella (0.72) > A. compressus (0.83) = T. involuta (0.83) at 400 mM NaCl The correlation between physiological and biochemical parameters of these five turfgrasses was also assessed, with a strong correlation with MDA content in T. involuta, with proline content and POD, SOD activity in L. repens and Z. matrella, with proline content and CAT, SOD activity in C. dactylon, and with proline content and POD activity in A. compressus, suggesting their different roles in the protective mechanism underlying different concentrations of salt stress tolerance.
References
Abogadallah GM (2010) Antioxidative defense under salt stress. Plant Signal Behav 5:369–374. https://doi.org/10.4161/psb.5.4.10873
Adams J, Samimi C, Mitterer C, Bendix J, Beck E (2022) Comparison of pasture types in the tropical andes: species composition, distribution, nutritive value and responses to environmental change. Basic Appl Ecol 59:139–150. https://doi.org/10.1016/j.baae.2022.01.005
Adomako MO, Yu FH (2023) Effects of resource availability on the growth, cd accumulation, and photosynthetic efficiency of three hyperaccumulator plant species. J Environ Manage 345:118762. https://doi.org/10.1016/j.jenvman.2023.118762
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23:731–744. https://doi.org/10.1007/s12298-017-0462-7
Ali A, Yun DJ (2017) Salt stress tolerance; what do we learn from halophytes? J Plant Biol 60:431–439. https://doi.org/10.1007/s12374-017-0133-9
Bendaly A, Messedi D, Smaoui A, Ksouri R, Bouchereau A (2016) Physiological and leaf metabolome changes in the xerohalophyte species Atriplex Halimus induced by salinity. Plant Physiol Biochem 103:208–218. https://doi.org/10.1016/j.plaphy.2016.02.037
Borsani O, Valpuesta V, Botella MA (2003) Developing salt tolerant plants in a new century: a molecular biology approach. Plant Cell Tiss Org Cult 73:101–115. https://doi.org/10.1023/A:1022849200433
Chai M, Jia Y, Chen S, Gao ZS, Wang HF, Liu LL, Wang PJ, Hou DQ (2011) Callus induction, plant regeneration, and long-term maintenance of embryogenic cultures in Zoysia Matrella [L.] Merr. Plant Cell Tiss Org Cult 104:187–192. https://doi.org/10.1007/s11240-010-9817-2
Chen SM, Zhang CM, Peng H, Qin YY, Li L, Li CG, Xing K, Liu LL, Qin S (2023a) Exopolysaccharides from endophytic Glutamicibacter halophytocota KLBMP 5180 functions as bio-stimulants to improve tomato plants growth and salt stress tolerance. Int J Biol Macromol 253:126717. https://doi.org/10.1016/j.ijbiomac.2023.126717
Chen X, Min D, Yasir TA, Hu Y (2012) Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res 137:195–201. https://doi.org/10.1016/j.fcr.2012.09.008
Chen Y, Li L, Zong J, Chen J, Guo H, Guo A, Liu J (2015a) Heterologous expression of the halophyte Zoysia Matrella H+-pyrophosphatase gene improved salt tolerance in Arabidopsis Thaliana. Plant Physiol Biochem 91:49–55. https://doi.org/10.1016/j.plaphy.2015.04.004
Chen Y, Zhou X, Wang Z, Su X, Liu F, Tian X, Ye Y, Shao Y, Yuan Z (2023b) Cd contamination determined assembly processes and network stability of AM fungal communities in an urban green space ecosystem. Sci Total Environ 899:166372. https://doi.org/10.1016/j.scitotenv.2023.166372
Chen Y, Zong J, Tan Z, Li L, Hu B, Chen C, Chen J, Liu J (2015b) Systematic mining of salt-tolerant genes in halophyte - Zoysia Matrella through cDNA expression library screening. Plant Physiol Biochem 89:44–52. https://doi.org/10.1016/j.plaphy.2015.02.007
Chuks KO, Nenibarini Z, Kabari S, Chibuzor NE (2019) Status, progress and challenges of phytoremediation - An African scenario. J Environ Manage 237:365–378. https://doi.org/10.1016/j.jenvman.2019.02.090
Custódio L, Charles G, Magné C, Barba-Espín G, Piqueras A, Hernández JA, Ben Hamed K, Castañeda-Loaiza V, Fernandes E, Rodrigues MJ (2023) Application of in vitro plant tissue culture techniques to halophyte species: A review. Plants 12:126. https://doi.org/10.3390/plants12010126
Dhandapani M, Hong SB, Aswath CR, Kim DH (2008) Regeneration of Zoysia grass (Zoysia Matrella L. Merr.) cv. Konhee from young inflorescences and stem nodes. Vitro Cell Dev Biol Plant 44:8–13. https://doi.org/10.1007/s11627-006-9021-6
Duan H, Ma Y, Liu R, Li Q, Yang Y, Song J (2018) Effect of combined waterlogging and salinity stresses on euhalophyte Suaeda glauca. Plant Physiol Biochem 127:231–237. https://doi.org/10.1016/j.plaphy.2018.03.030
El-Amier YA, Soufan W, Almutairi KF, Zaghloul NS, Abd-ElGawad AM (2022) Proximate composition, bioactive compounds, and antioxidant potential of wild halophytes grown in coastal salt marsh habitats. Molecules 27:28. https://doi.org/10.3390/molecules27010028
Flowers TJ, Hajibagheri MA, Clipson NJW (1986) Halophytes. Quart Rev Biol 61:313–337. https://hdl.handle.net/10779/uos.23384564.v1
Ghars MA, Parre E, Debez A, Richard L, Leport L, Bouchereau A, Savoure A, Abdelly C (2008) Comparative salt tolerance analysis between Arabidopsis Thaliana and Thellungiella halophila, with special emphasis on K+ /Na+ selectivity and proline accumulation. J Plant Physiol 165:588–599. https://doi.org/10.1016/j.jplph.2007.05.014
Greenway H, Munns RA (1980) Mechanisms of salt tolerance in nonhalophytes. Ann Rev Plant Biol 31:149–190. https://doi.org/10.1146/annurev.pp.31.060180.001053
Guan B, Yu J, Wang X, Fu Y, Kan X, Lin Q, Han G, Lu Z (2011) Physiological responses of halophyte Suaeda Salsa to water table and salt stresses in coastal wetland of yellow river delta. Clean Soil Air Water 39:1029–1035. https://doi.org/10.1002/clen.201000557
Hadjadj S, Mahdjoubi S, Hidoub Y, Bahaz T, Ghedamsi Z, Regagda S, Arfa Y, Hadj-Khelil AOE (2023) Comparative effects of NaCl and Na2SO4 on germination and early seedling stages of the halophyte Carthamus tinctorius L. J Appl Res Med Arom Plants 35:100463. https://doi.org/10.1016/j.jarmap.2023.100463
Hameed M, Ashraf M (2008) Physiological and biochemical adaptations of Cynodon dactylon (L.) pers. From the salt range (Pakistan) to salinity stress. Flora 203:683–694. https://doi.org/10.1016/j.flora.2007.11.005
Hashemipetroudi SH, Ahmadian G, Fatemi F, Nematzadeh G, Yamchi A, Kuhlmann M (2022) Ion content, antioxidant enzyme activity and transcriptional response under salt stress and recovery condition in the halophyte grass Aeluropus littoralis. BMC Res Notes 15:201. https://doi.org/10.1186/s13104-022-06090-4
Hassan MA, Pacurar A, López-Gresa MP, Donat-Torres MP, Vicente O (2016) Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 11:e0160236. https://doi.org/10.1371/journal.pone.0160236
Hu Y, Yue J, Nie J, Luo D, Cao S, Wang C, Pan J, Chen C, Zhang H, Wu Q, Tan Y, Li R, Chen P (2023) Salicylic acid alleviates the salt toxicity in Kenaf by activating antioxidant system and regulating crucial pathways and genes. Ind Crop Prod 199:116691. https://doi.org/10.1016/j.indcrop.2023.116691
Ibeh B, Maxwell E, Bitrus JH (2013) Phytochemical compositions and in vitro antioxidant capacity of methanolic leaf extract of Axonopus compressus (P. Beauv). Eur J Med Plants 3:254–265. https://doi.org/10.9734/ejmp/2013/1686
Jithesh MN, Prashanth SR, Sivaprakash KR, Parida AK (2006) Antioxidative response mechanisms in halophytes: their role in stress defense. J Genet 85:237–254. https://doi.org/10.1007/BF02935340
Kumari A, Das P, Parida AK, Agarwal PK (2015) Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front Plant Sci 6:537. https://doi.org/10.3389/fpls.2015.00537
Li D, Liu J, Guo H, Zong J, Li J, Wang J, Li L, Chen J (2022a) Effects of low nitrogen supply on nitrogen uptake, assimilation and remobilization in wild Bermudagrass. Plant Physiol Bioch 191:34–41. https://doi.org/10.1016/j.plaphy.2022.09.019
Li F, Jin H, Wu X, Liu Y, Chen X, Wang J (2022b) Remediation for trace metals in polluted soils by turfgrass assisted with chemical reagents. Chemosphere 295:133790. https://doi.org/10.1016/j.chemosphere.2022.133790
Li H, Wang H, Wen W, Yang G (2020) The antioxidant system in Suaeda Salsa under salt stress. Plant Signal Behav 15:1771939. https://doi.org/10.1080/15592324.2020.1771939
Li L, Qu R (2004) Development of highly regenerable callus lines and biolistic transformation of turf-type common Bermudagrass (Cynodon dactylon L. Pers). Plant Cell Rep 22:403–407. https://doi.org/10.1007/s00299-003-0706-6
Li RF, Wei JH, Wang HZ, He J, Sun ZY (2006) Development of highly regenerable callus lines and Agrobacterium-mediated transformation of Chinese lawngrass (Zoysia sinica Hance) with a cold inducible transcription factor, cbf1. Plant Cell Tiss Org Cult 85:297–305. https://doi.org/10.1007/s11240-006-9080-8
Li X, Ye G, Shen Z, Li J, Hao D, Kong W, Wang H, Zhang L, Chen J, Guo H (2023) Na+ and K+ homeostasis in different organs of contrasting Zoysia Japonica accessions under salt stress. Environ Exp Bot 214:105455. https://doi.org/10.1016/j.envexpbot.2023.105455
Liu L, Fang XL, Zhang JW, Bao YM, Yan M (2009) Long-term cultured callus and the effect factor of high-frequency plantlet regeneration and somatic embryogenesis maintenance in Zoysia Japonica. Vitro Cell Dev Biol - Plant 45:673–680. https://doi.org/10.1007/s11627-009-9226-6
Liu M, Sun T, Liu C, Zhang H, Wang W, Wang Y, Xiang L, Chan Z (2022) Integrated physiological and transcriptomic analyses of two warm- and cool-season turfgrass species in response to heat stress. Plant Physiol Biochem 170:275–286. https://doi.org/10.1016/j.plaphy.2021.12.013
Lu SY, Wang ZC, Peng XX, Guo Z, Zhang G, Han L (2006) An efficient callus suspension culture system for triploid Bermuda grass (Cynodon transvaalensis × C. dactylon) and Somaclonal variations. Plant Cell Tiss Org Cult 87:77–84. https://doi.org/10.1007/s11240-006-9138-7
Ma GH, Jian SG, Ren H (eds) (2021) Plant Proliferation and Cultivation Techniques for Tropical Island Reefs. China Forestry Press, Beijing, pp 7–17. ISBN: 978-7-5219-0955-5
Mallik S, Nayak M, Sahu BB, Panigrahi AK, Shaw BP (2011) Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biol Plant 55:191–195. https://doi.org/10.1007/s10535-011-0029-3
Manuchehri R, Salehi H (2014) Physiological and biochemical changes of common Bermudagrass (Cynodon dactylon [L.] Pers.) under combined salinity and deficit irrigation stresses. S Afr J Bot 92:83–88. https://doi.org/10.1016/j.sajb.2014.02.006
McElwee P, Calvin K, Campbell D, Cherubini F, Grassi G, Korotkov V (2020) The impact of interventions in the global land and agri-food sectors on nature’s contributions to people and the UN sustainable development goals. Global Change Biol 26:4691–4721. https://doi.org/10.1111/gcb.15219
Mishra A, Tanna B (2017) Halophytes: potential resources for salt stress tolerance genes and promoters. Front Plant Sci 8:829. https://doi.org/10.3389/fpls.2017.00829
Muchate NS, Nikalje GC, Rajurkar GC, Suprasanna NS, Nikam P, Tukaram D (2016) Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot Rev 82:371–406. https://doi.org/10.1007/s12229-016-9173-y
Nawaz M, Wang Z (2020) Abscisic acid and Glycine betaine mediated tolerance mechanisms under drought stress and recovery in Axonopus compressus: a new insight. Sci Rep 10:6942. https://doi.org/10.1038/s41598-020-63447-0
Niu X, Bressan RA, Hasegawa PM, Pardo JM, Niu XM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742 PMID: 12228628
Ozgur R, Uzilday B, Sekmen AH, Turkan I (2013) Reactive oxygen species regulation and antioxidant defence in halophytes. Funct Plant Biol 40:832–847. https://doi.org/10.1071/FP12389
Parida AK, Jha B (2010) Salt tolerance mechanisms in mangroves: a review. Trees 24:199–217. https://doi.org/10.1007/s00468-010-0417-x
Parthasarathy M, Pemaiah B, Natesan R, Padmavathy SR, Pachiappan J (2015) Real-time mapping of salt glands on the leaf surface of Cynodon dactylon L. using scanning electrochemical microscopy. Bioelectrochemistry 101:159–164. https://doi.org/10.1016/j.bioelechem.2014.10.004
Rasheed R, Wahid A, Hussain I, Mahmood S, Parveen A (2016) Partial repair of salinity-induced damage to sprouting sugarcane buds by proline and Glycine betaine pretreatment. Protoplasma 253:803–813. https://doi.org/10.1007/s00709-015-0841-2
Ren H, Jian SG, Zhang QM, Wang FG, Shen T, Wang J (2017) Plants and vegetation on South China sea Islands. Ecol Environ Sci 26:1639–1648. http://www.jeesci.com
Roy S, Chakraborty U (2014) Salt tolerance mechanisms in salt tolerant grasses (STGs) and their prospects in cereal crop improvement. Bot Stud 55:1–9. https://doi.org/10.1186/1999-3110-55-31
Ruan CJ, Jaime A, Teixeira da Silva (2011) Metabolomics: creating new potentials for unraveling the mechanisms in response to salt and drought stress and for the biotechnological improvement of xero-halophytes. Crit Rev Biotech 31:153–169. https://doi.org/10.3109/07388551.2010.505908
Rupasinghe HT, Halwatura RU (2020) Benefits of implementing vertical greening in tropical climates. Urban Urban Green 53:126708. https://doi.org/10.1016/j.ufug.2020.126708
Sahu BB, Shaw BP (2009) Isolation, identification and expression analysis of salt-induced genes in Suaeda maritima, a natural halophyte, using PCR-based suppression subtractive hybridization. BMC Plant Biol 9:69. https://doi.org/10.1186/1471-2229-9-69
Singh PK, Indoliya Y, Agrawal L, Awasthi S, Deeba F, Dwivedi S, Chakrabarty D, Shirke PA, Pandey V, Singh N, Dhankher OP, Barik SK, Tripathi RD (2022) Genomic and proteomic responses to drought stress and biotechnological interventions for enhanced drought tolerance in plants. Curr Plant Biol 29:100239. https://doi.org/10.1016/j.cpb.2022.100239
Souid A, Bellani L, Tassi EL, Ben Hamed K, Longo V, Giorgetti L (2023) Early physiological, cytological and antioxidative responses of the edible halophyte Chenopodium Quinoa exposed to salt stress. Antioxidants 12:1060. https://doi.org/10.3390/antiox12051060
Turner NC, Colmer TD, Quealy J, Pushpavalli R, Krishnamurthy L, Kaur J, Singh G, Siddique KHM, Vadez V (2013) Salinity tolerance and ion accumulation in Chickpea (Cicer arietinum L.) subjected to salt stress. Plant Soil 365:347–361. https://doi.org/10.1007/s11104-012-1387-0
Volkov V, Amtmann A (2006) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+ /Na+ homeostasis under salinity stress. Plant J 48:342–353. https://doi.org/10.1111/j.1365-313X.2006.02876.x
Wang B, Luttge U, Ratajczak R (2004) Specific regulation of SOD isoforms by NaCl and osmotic stress in leaves of the C3 halophyte Suaeda Salsa L. J Plant Physiol 161:285–293. https://doi.org/10.1078/0176-1617-01123
Wang H, Li J, Liu H, Chen S, Zaman Q, Rehman M, El-Kahtany K, Fahad S, Deng G, Yang J (2023) Variability in morpho-biochemical, photosynthetic pigmentation, enzymatic and quality attributes of potato for salinity stress tolerance. Plant Physiol Bioch 203:108036. https://doi.org/10.1016/j.plaphy.2023.108036
Wang J, Meng Y, Li B, Ma X, Lai Y, Si E, Yang K, Xu X, Shang X, Wang H, Wang D (2015) Physiological and proteomic analyses of salt stress response in the halophyte Halogeton glomeratus. Plant Cell Environ 38:655–669. https://doi.org/10.1111/pce.12428
Wang Y, Tan SN, Yusof MLM, Ghosh S, Lam YM (2022) Assessment of heavy metal and metalloid levels and screening potential of tropical plant species for phytoremediation in Singapore. Environ Poll 295:118681. https://doi.org/10.1016/j.envpol.2021.118681
Wei ZP, Xiong YP, Zeng YJ, Liu JY, Jian SG, Wu KL, Zeng SJ, Jaime A, Teixeira da Silva, Ma GH (2023) Protocol for shoot proliferation and regeneration of a salt-tolerant plant, Thuarea involuta. Plant Cell Tiss Org Cult 154:201–207. https://doi.org/10.1007/s11240-023-02531-5
Xiong YP, Wei ZP, Yu XC, Pang JH, Zhang T, Wu KL, Ren H, Jian SG, Jaime A, Teixeira da Silva, Ma GH (2021) Shoot proliferation, embryogenic callus induction, and plant regeneration in Lepturus repens (G. Forst.) R. Br. Vitro Cell Dev Biol - Plant 57:1031–1039. https://doi.org/10.1007/s11627-021-10183-3
Xiong YP, Wei ZP, Liu JY, Li JR, Jian SG, Zhang XH, Li Y, Bian Z, Wu KL, Zeng SJ, Jaime A. Teixeira da Silva, Ma GH (2024) Shoot propagation, regeneration, and callus induction and differentiation, of Axonopus compressus (Swartz) Beauv. In Vitro Cell Dev Biol – Plant 60:478–486. https://doi.org/10.1007/s11627-024-10432-1
Author Information
Guangdong Provincial Key Laboratory of Applied Botany, Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China