Influence and damage potential of cercospora leaf spot disease and root-knot nematode, Meloidogyne incognita on plant growth parameters of Vigna radiata
Research Articles | Published: 27 January, 2023
First Page: 133
Last Page: 143
Views: 3304
Keywords: n Cercospora canescensn , Cercospora leaf spot disease, Nematode, n Meloidogyne incognitan , Mungbean, n Vigna radiatan
Abstract
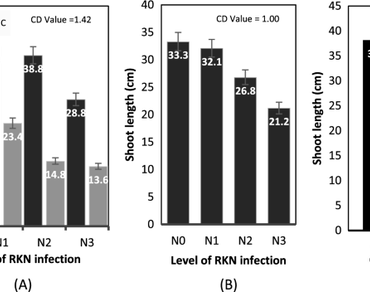
Mungbean, Vigna radiata, is a very important short period legume crop of summer and monsoon season. Among the various diseases associated with mungbean, root-knot nematode Meloidogyne spp. and Cercospora canescens fungus leaf spot are significant limiting factors in effective cultivation of crop. The plants are affected by cercospora or root-knot nematode separately or by both the pathogens. This study revealed a progressive decline in growth parameters of plant viz., length of shoot and root, fresh and dry weight of plant, leaf surface area and chlorophyll content were affected by the Cercospora leaf spot fungus and increasing inoculation levels of plant parasitic root-knot nematode, Meloidogyne incognita, individually and together. There were significant interaction effects in combined infections of root-knot nematode and leaf spot disease. Rate of reproduction of the nematode decreased with increase in inoculation level and also in the presence of Cercospora leaf spot disease.
References
Abad PB, Favery MN, Rosso P, Castagnone-Sereno P (2003) Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol Plant Pathol 4:217–224. https://doi.org/10.1046/j.1364-3703.2003.00170.x
Ahmed N, Abbasi MW, Shaukat SS, Zaki MJ (2009) Physiological changes in leaves of mungbean plants infected with Meloidogyne javanica. Phytopathologia mediterranea 48:262–268. https://www.jstor.org/stable/26463350
Ali N, Tavoillot J, Mateille T, Chapuis E, Besnard G, El Bakkali A, Palomares-Rius JE (2015) A new root-knot nematode Meloidogyne spartelensis n. sp. (Nematoda: Meloidogynidae) in Northern Morocco. Eur J Plant Pathol 143:25–42. https://doi.org/10.1007/s10658-015-0662-3
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Barbetti MJ (1985) Infection studies with Cercospora zebrina on pasture legumes in Western Australia. Aust J Exp Agric 25:850–855
Barker KR (1985) Nematode extraction and bioassays. In: Barker, Carter and Sasser (eds) An advanced treatise on Meloidogyne Vol 2. Methodology. North Carolina State University Graphics, Raleigh, USA, p 19–35
Bernard GC, Egnin M, Bonsi C (2017) The impact of plant parasitic nematodes on agriculture and methods of control. Nematol-Concepts, Diagn Control 10:121–127. https://doi.org/10.5772/intechopen.68958
Bhagwati B, Phukan PN (1991) Pathogenicity of root-knot nematode, Meloidogvne incognita on pea. Indian J Nematol 21:141–144
Bybd DW Jr, Kirkpatrick T, Barker K (1983) An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol 15:142–143
Chahal PPK, Chahal VPS (1987) Adverse effect of Meloidogyne incognita on the functioning of nodules of mungbean (Vigna radiata). Nematol Mediterr 15:13–19
Chand R, Singh V, Pal C, Kumar P, Kumar M (2012) First report of a new pathogenic variant of Cercospora canescens on mungbean (Vigna radiata) from India. New Dis Rep 26:6. https://doi.org/10.5197/j.2044-0588.2012.026.006
Daub ME, Ehrenshaft M (2000) The photoactivated Cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu Rev Phytopathol 38:461–490
Davis EL, Mitchum MG (2005) Nematodes sophisticated parasites of legumes. Plant Physiol 137:1182–1188. https://doi.org/10.1104/pp.104.054973
Devi ML, Kumari MV (2014) Prevalence of Meloidogyne species in different crops of Indian sub-continent-a review. Int J Adv Res 2:533–546
Eisenback JD, Hirschmann H, Triantaphyllou AC (1980) Morphological comparison of Meloidogyne female head structures, perineal patterns, and stylets. J Nematol 12:300
Ellis JB, Martin GB (1882) Macrosporium solani E&M. Am Nat 16:1003
Esbenshade PR, Triantaphyllou AC (1985) Use of enzyme phenotypes for identification of Meloidogyne species. J Nematol 17:6
Gaur HS, Von MN, Perry RN (1996) Differentiation of two groups of species of the genus Meloidogyne by polymerase chain reaction and restriction fragment length polymorphisms of ribosomal DNA. Afroasian J Nematol 6:50–54
Grewal JS, Pal M, Kulshreshtha DD (1980) Control of Cercospora leaf spots of greengram by spraying Bavistin. Indian J Agric Sci 50:707–711
Gupta DC, Paruthi IJ, Verma KK (1986) Reaction of mung bean germplasm and its pathogenicity against Meloidogyne javanica. Indian J Nematol 16:194–196
Haider MG, Dev LK, Nath RP (2003) Comparative pathogenicity of root-knot nematode Meloidogyne incognita on different pulse crops. Indian J Nematol 33:152–153
Hartman KM, Sasser JN (1985) Identification of Meloidogyne species on the basis of differential host test and perineal pattern morphology. In: Barker KR, Carter CC Sasser JN (eds) An advanced treatise on meloidogyne. Vol 2 Methodology. A cooperative publication of the Department of Plant Pathology and the United States Agency for International Development, North Carolina State University Graphics, Raleigh, pp 69–77
Hisamuddin SS, Azam T (2010) Pathogenicity of root-knot nematode, Meloidogyne incognita on Medicago truncatula (Medik.). Arch Phytopathol Plant Prot 43:1504–1511. https://doi.org/10.1080/03235400802583537
Hussain MA, Mukhtar T, Kayani MZ (2011) Assessment of the damage cause by Meloidogyne incognita on okra (Abelmoschus esculentus). J Anim Plant Sci 21:857–861
Hussaini SS, Seshadri AR (1975) Interrelationship between Meloidogyne incognita and Rhizobium sp. on mung (Phaseolus aureus). Indian J Nematol 5:189–199
Iqbal SM, Ghafoor A, Bashir M, Malik BA (1995) Estimation of losses in yield components of mungbean due to Cercospora leaf spot. Pak J Phytopathol 7:80–81
Jain HK, Mehra KL (1980) Evolution, adaptation, relationships, and uses of the species of Vigna cultivated in India. Evolution, adaptation, relationships, and uses of the species of Vigna cultivated in India 459–468
Jamadar MM (1988) Studies on leaf spot of greengram (Vigna radiate L.) Wilczek caused by Cercospora canescens Ell. and Mart. M.Sc. (Agri.) Thesis, Univ Agri Sci Dharwad
Jones JG, Kleczewski NM, Desaeger J, Meyer SL, Johnson GC (2017) Evaluation of nematicides for southern root-knot nematode management in lima bean. Crop Prot 96:151–157. https://doi.org/10.1016/j.cropro.2017.02.015
Joshi V, Kumar S, Rawat S (2020) Study on infection and development of root-knot nematode, Meloidogyne javanica on mungbean. J Entomol and Zool Stud 8:1621–1626
Kalita DN, Phukan PN (1993) Pathogenicity of Meloidogyne incognita on blackgram. Indian J Nematol 23:105–109
Kasno A (1990) The tolerance of mungbean genotypes to Cercospora sp. leaf spot. Penelitian Palawija (Indonesia) 5:39–47
Kaur L (2006) Multiple disease resistant sources of mungbean , In I International Conference on Indigenous Vegetables and Legumes. Prospectus Fighting Poverty, Hunger Malnutrition 752:423–426. https://doi.org/10.17660/ActaHortic.2007.752.76
Kayani MZ, Mukhtar T, Hussain MA (2017) Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Prot 92:207–212. https://doi.org/10.1016/j.cropro.2016.09.007
Keatinge JDH, Easdown WJ, Yang RY, Chadha ML, Shanmugasundaram S (2011) Overcoming chronic malnutrition in a future warming world: the key importance of mung bean and vegetable soybean. Euphytica 180:129–141. https://doi.org/10.1007/s10681-011-0401-6
Khan MR, Kounsar K, Hamid A (2002) Effect of certain rhizobacteria and antagonistic fungi on root nodulation and root-knot nematode disease of green gram. Nematol Mediterr 30:85–89
Kumar D, Bhatt J, Sharm RL (2018) Effect of different inoculum levels, plant age and multiplication of Meloidogyne incognita on growth of blackgram (Vigna mango L). J Entomol Zool Stud 6:452–455
Kumar R, Dhillon NK, Kaur S, Anupam SA (2020) Resistance in Mungbean against Meloidogyne incognita and its Impact on nodulation. Legume Res-Int J 44:1293–1300
Msuku WAB, Saka VW, Mbalule JC, Kamwanba S (1990) Evaluation of bean (Phaseolus vulgaris) germplasm against angular leaf spot and some root knot nematode (Meloidogyne) species in Malawi. Zimb J Agric Res 28:15–21
Munjal RL, Lal G, Chona BL (1960) Some Cercospora species from India-IV. Indian Phytopatholol 13:144–149
Pande S, Sharma M, Kumari S, Gaur PM, Chen W, Kaur L, et al. (2009) “Integrated foliar diseases management of legumes,” In International Conference on Grain Legumes: Quality Improvement, Value Addition and Trade (Kanpur: Indian Society of Pulses Research and Development, Indian Institute of Pulses Research), 143–161
Pazarlar S, Gümüş M, Öztekin GB (2013) The effects of tobacco mosaic virus infection on growth and physiological parameters in some pepper varieties (Capsicum annuum L.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 41:427–433. https://doi.org/10.15835/nbha4129008
Perveen K, Haseeb A, Shukla PK (2006) Pathogenic potential of Meloidogyne incognita on Mentha arvensis cv. Gomti. Indian J Nematol 36:157–160
Poehlman JM (1991) The Mungbean. Westview Press, Boulder, pp 169–274
Poudyal DS, Pokharel RR, Shrestha SM, Khatri-ChetriA GB (2005) Effect of inoculum density of rice root knot nematode on growth of rice cv. Masuli and nematode development. Australas Plant Pathol 34:181–185. https://doi.org/10.1071/AP05011
Pratap A, Douglas C, Prajapati U, Kumari G, War AR, Tomar R, Pandey AK, Dubey S (2020) Breeding progress and future challenges: biotic stresses. In The mungbean genome. Springer, Cham, pp 55–80
San Chin SL (2019) The Role of flavonoids in the interaction between the plant, Medicago truncatula and the Nematode, Meloidogyne javanica (Doctoral dissertation, The Australian National University (Australia)
Sarathchandra SU, Di Menna ME, Burch G, Brown JA, Watson RN, Bell NL, Cox NR (1995) Effects of plant-parasitic nematodes and rhizosphere microorganisms on the growth of white clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.). Soil Biol Biochem 27:9–16
Sasser JN, Freckman DW (1987) A world perspective on nematology: The role of the Society. In: Vistas on Nematology, Hyattsville, Maryland, pp. 7–14
Schindler A (1961) A simple substitute for a Baermann funnel. Plant Dis Rep 45:747–749
Seinhorst JW (1967) The relationships between population increase and population density in plant parasitic nematodes: I., definitions of the terms host, host status and resistance: the influence of external conditions on the regulation of population density. Nematologica 13:429–450. https://doi.org/10.1163/187529267X00670
Sharma SB, Sharma HK, Pankaj (2000) Nematode problem in India, In: Prasad D, Puri SN (eds). Crop pest and disease management-challenges for the millennium. Jyoti Publishers, New Delhi pp 267-275
Singh DB, Reddy PP (1981) Influence of M. incognita infestation on rhizobium nodule formation in French bean. Nematol Mediterr 9:1–5
Tabreiz AK, Iram TA, Shweta S (2012) Comparative studies on the pathogenic potential of Meloidogyne spp. on mung bean (Vigna radiata L.). African J Microbiol Res 6:7134–7138. https://doi.org/10.5897/AJMR11.1053
Taylor AL, Sasser JN (1978) Biology, identification and control of root-knot nematodes. North Carolina State University Graphics, North Carolina, pp 111–114
Vakili NG (1977) Field screening of cowpeas for Cercospora leaf spot resistance. Trop Agric 54:69–76
Vasudeva RS (1963) Indian Cercosporae, I.C.A.R. Publication, 245
Vavilov NI (1951) The origin, variation, immunity, and breeding of cultivated plants. (Translation by K.S. Chester). Chron Bot 13:1–364
Verdcourt B (1970) Studies in the Leguminosae-Papilionoïdeae for the ‘Flora of tropical East Africa’: III. Kew Bulletin, pp 379–447
Waghmare C, Singh P, Paul S, Sharma HK (2022) Influence of root-knot nematode, Meloidogyne incognita (Kofoid & White) Chitwood infection on different plant growth parameters in Mungbean, Vigna radiata (L.) Wilczek. Indian J Exp Biol (IJEB) 60:351–359
Wilczek R (1954) Vigna. In: Fiore du Congo Beige 6:343-393
Windels CE, Lamay HA, Hilde D, Winder J, Knudsen TA (1998) Cercospora leaf spot model for sugar beet. Plant Dis 82:716–726. https://doi.org/10.1094/PDIS.1998.82.7.716
Zijlstra C, Donkers-Venne DTHM, Fargette M (2000) Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology 2:847–853
Author Information
School of Basic Sciences and Research, Sharda University, Greater Noida, India