Isolation and Identification of antifungal metabolite producing endophytic Bacillus subtilis (S17) and its in vitro effect on Colletotrichum falcatum causing red rot in sugarcane
Research Articles | Published: 06 July, 2020
First Page: 493
Last Page: 503
Views: 4064
Keywords: Sugarcane, Red rot, Colletotrichum falcatum Went, Endophyte, Bacillus subtilis , Lytic enzyme
Abstract
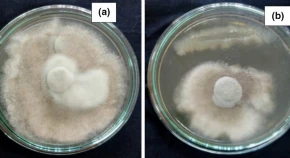
Red rot, a threatening disease of sugarcane caused by fungal pathogen Colletotrichum falcatum Went adversely affect the yield and quality of crop. Therefore, it is important to explore such methods of protection which are organically safe especially with the use of bioagents. Currently, usage of endophytic bacteria as biocontrol agent have been focussed in the field of biological control and enhancement of crop productivity. Hence, this study was centred on endophytic bacteria Bacillus subtilis isolate S17 (identified by 16S rRNA analysis) isolated from the sugarcane stalk tissues. The in vitro study indicated that isolate S17 inhibited the mycelial growth of C. falcatum by 76.22 ± 2.34% in dual-culture antagonism assay. Scanning electron microscopy (SEM) revealed the distortion and destruction in the hyphae of the fungal pathogen by isolate S17. Further investigations proved that the hyphal disorientation was by the secretion of various enzymes (chitinase, β-1,3 glucanase, protease, pectinase, and amylase), inhibitory substances viz., siderophore, ammonia, hydrogen cyanide (HCN) and other volatile as well as diffusible metabolites. The volatile and diffusible metabolites exhibited strong inhibition (74.57 ± 1.45% and 81.86 ± 2.36%) against C. falcatum, respectively. Similarly, cell-free culture supernatant that contains extracellular metabolites exhibited mycelial inhibition (70.52 ± 0.95%) against C. falcatum. Thus, the present finding revealed that different metabolites as well as various exo-enzymes produced by B. subtilis (S17) inhibit red rot pathogen besides mechanism of the antagonism.
References
- Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971
- Baldan ES, Nigris C, D’Alessandro RS, Clocchiatti A, Zottini M, Stevanato P, Squartini A, Baldan B (2015) Beneficial bacteria isolated from grapevine inner tissues shape Arabidopsis thaliana roots. PLoS ONE 10:1–18
- Chaurasia B, Pandey A, Palni LM, Trivedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 160:75–81
- Chen Y, Zhou MG (2009) Characterization of Fusarium graminearum isolates resistant to both carbendazim and a new fungicide JS399-19. Phytopathology 99:441–446
- Cho ST, Chang HH, Egamberdieva D, Kamilova F, Lugtenberg B, Kuo CH (2015) Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE 10:1–16
- Cordero P, Príncipe A, Jofré E, Mori G, Fischer S (2014) Inhibition of the phytopathogenic fungus Fusarium proliferatum by volatile compounds produced by Pseudomonas. Arch Microbiol 196:803–809
- De Boer W, Gunnewiek PJ, Lafeber P, Janse JD, Spit BE, Woldendorp JW (1998) Anti-fungal properties of chitinolytic dune soil bacteria. Soil Biol Biochem 30:193–203
- Deb P, Talukdar SA, Mohsina K, Sarker PK, Sayem SA (2013) Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. Springer Plus 2:154
- Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, Abd Allah EF (2017) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol 8:1–13
- Fang H, Wang Y, Gao C, Yan H, Dong B, Yu Y (2010) Isolation and characterization of Pseudomonas sp. CBW capable of degrading carbendazim. Biodegradation 21:939–946
- Fravel DR (1988) Role of antibiosis in the biocontrol of plant diseases. Annu Rev Phytopathol 26:75–91
- Gajbhiye A, Rai AR, Meshram SU, Dongre AB (2010) Isolation, evaluation and characterization of Bacillus subtilis from cotton rhizospheric soil with biocontrol activity against Fusarium oxysporum. World J Microbiol Biotechnol 26:1187–1194
- Gao Z, Zhang B, Liu H, Han J, Zhang Y (2017) Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol control 105:27–39
- Gessesse A, Gashe BA (1997) Production of alkaline xylanase by an alkaliphilic Bacillus sp. isolated from an alkaline soda lake. J Appl Microbiol 83:402–406
- Han JH, Shim H, Shin JH, Kim KS (2015) Antagonistic activities of Bacillus spp. strains isolated from tidal flat sediment towards anthracnose pathogens Colletotrichum acutatum and C. gloeosporioides in South Korea. Plant Pathol J 31:165–175
- Haran S, Schickler H, Chet I (1996) Molecular mechanisms of lytic enzymes involved in the biocontrol activity of Trichoderma harzianum. Microbiology 142:2321–2331
- Hassan MN, Afghan S, Hafeez FY (2010) Suppression of red rot caused by Colletotrichum falcatum on sugarcane plants using plant growth-promoting rhizobacteria. Biocontrol 55:531–542
- Hassan MN, Shah SZ, Afghan S, Hafeez FY (2015) Suppression of red rot disease by Bacillus sp. based biopesticide formulated in non-sterilized sugarcane filter cake. Biocontrol 60:691–702
- Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. A Waverly Company Williams and Wilkins, Baltimore
- ISMA (2019). Indian sugar mill association; https://www.indiansugar.com/Statics.aspx
- Islam MA, Nain Z, Alam MK, Banu NA, Islam MR (2018) In vitro study of biocontrol potential of rhizospheric Pseudomonas aeruginosa against Fusarium oxysporum f. sp. cucumerinum. Egypt J Biol Pest Control 28:90
- Jahangir GZ, Sadiq M, Hassan N, Nasir IA, Saleem MZ, Iqbal M (2016) The effectiveness of phosphate solubilizing bacteria as biocontrol agents. J Anim Plant Sci 26:1313–1319
- Jasim B, Joseph AA, John CJ, Mathew J, Radhakrishnan EK (2014) Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech 4:197–204
- Ji SH, Gururani MA, Chun SC (2014) Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res 169:83–98
- Kalayu G (2019) Phosphate solubilizing microorganisms: promising approach as biofertilizers. Intern J Agron 2019:1–7
- Kandel SL, Firrincieli A, Joubert PM, Okubara PA, Leston ND, McGeorge KM, Mugnozza GS, Harfouche A, Kim SH, Doty SL (2017) An in vitro study of bio-control and plant growth promotion potential of Salicaceae endophytes. Front Microbiol 8:386
- Khan N, Martínez-Hidalgo P, Ice TA, Maymon M, Humm EA, Nejat N, Sanders ER, Kaplan D, Hirsch AM (2018) Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol 9:1–12
- Khan N, Maymon M, Hirsch A (2017) Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms 5:75
- Khatri DK, Tiwari DN, Bariya HS (2017) Chitinolytic efficacy and secretion of cell wall-degrading enzymes from Trichoderma spp. in response to phytopathological fungi. J Appl Biol Biotechnol 5:1–8
- Kim P, Chung KC (2004) Production of an antifungal protein for control of Colletotrichum lagenarium by Bacillus amyloliquefaciens MET0908. FEMS Microbiol Lett 234:177–183
- Kim YK, Hong SJ, Shim CK, Kim MJ, Choi EJ, Lee MH, Park JH, Han EJ, An NH, Jee HJ (2012) Functional analysis of Bacillus subtilis isolates and biological control of red pepper powdery mildew using Bacillus subtilis R2–1. Res Plant Dis 18:201–209
- Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4:317–320
- Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
- Kong WL, Li PS, Wu XQ, Wu TY, Sun XR (2020) Forest tree associated bacterial diffusible and volatile organic compounds against various phytopathogenic fungi. Microorganisms 8:590
- Kuddus M, Ahmad IZ (2013) Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Genet Eng Biotechnol 11:39–46
- Lahlali R, Peng G, Gossen BD, McGregor L, Yu FQ, Hynes RK, Hwang SF, McDonald MR, Boyetchko SM (2013) Evidence that the bio fungicide Serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology 103:245–254
- Lee GW, Kim MJ, Park JS, Chae JC, Soh BY, Ju JE, Lee KJ (2011) Biological control of Phytophthora blight and anthracnose disease in red-pepper using Bacillus subtilis S54. Res Plant Dis 17:86–89
- Lee SY, Lee SB, Kim YK, Hwang SJ (2006) Biological control of garlic white rot accused by Sclereotium cepivorum and Sclereotium sp. using Bacillus subtilis 122 and Trichoderma harzianum 23. Res Plant Dis 12:81–84
- Lee T, Park D, Kim K, Lim SM, Yu NH, Kim S, Kim HY, Jung KS, Jang JY, Park JC, Ham H (2017) Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathol J 33:499
- Maindad DV, Kasture VM, Chaudhari H, Dhavale DD, Chopade BA, Sachdev DP (2014) Characterization and fungal inhibition activity of siderophore from wheat rhizosphere associated Acinetobacter calcoaceticus strain HIRFA32. Indian J Microbiol 54:315–322
- Mardanova AM, Hadieva GF, Lutfullin MT, Khilyas IV, Minnullina LF, Gilyazeva AG, Bogomolnaya LM, Sharipova MR (2017) Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agri Sci 8:1–20
- Massawe VC, Hanif A, Farzand A, Mburu DK, Ochola SO, Wu L, Tahir HA, Gu Q, Wu H, Gao X (2018) Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology 108:1373–1385
- McDonald JE, Rooks DJ, McCarthy AJ (2012) Methods for the isolation of cellulose-degrading microorganisms. Methods Enzymol 510:349–374
- Mehnaz S, Saleem RS, Yameen B, Pianet I, Schnakenburg G, Pietraszkiewicz H, Valeriote F, Josten M, Sahl HG, Franzblau SG, Gross H (2013) Lahorenoic acids A-C, ortho-dialkyl-substituted aromatic acids from the biocontrol strain Pseudomonas aurantiaca PB-St2. J Nat Prod 76:135–141
- Mercado-Blanco J, Rodrıguez-Jurado D, Hervás A, Jiménez-Dıaz RM (2004) Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent Pseudomonas spp. Biol Control 30:474–486
- Montealegre JR, Reyes R, Pérez LM, Herrera R, Silva P, Besoain X (2003) Selection of bioantagonistic bacteria to be used in biological control of Rhizoctonia solani in tomato. Electr J Biotechnol 6:115–127
- Nandi M, Selin C, Brawerman G, Fernando WD, de Kievit T (2017) Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol Control 108:47–54
- Oyeleke SB, Oyewole OA, Egwim EC, Dauda BE, Ibeh EN (2012) Cellulase and pectinase production potentials of Aspergillus niger isolated from corn cob. Bayero J Pure Appl Sci 5:78–83
- Pandey G, Dorrian SJ, Russell RJ, Brearley C, Kotsonis S, Oakeshott JG (2010) Cloning and biochemical characterization of a novel carbendazim (methyl-1H-benzimidazol-2-ylcarbamate)-hydrolyzing esterase from the newly isolated Nocardioides sp. strain SG-4G and its potential for use in enzymatic bioremediation. Appl Environ Microbiol 76:2940–2945
- Park JW, Balaraju K, Kim JW, Lee SW, Park K (2013) Systemic resistance and growth promotion of chili pepper induced by an antibiotic producing Bacillus vallismortis strain BS07. Biol Control 65:246–257
- Parray JA, Kamili AN, Reshi ZA, Qadri RA, Jan S (2015) Interaction of rhizobacterial strains for growth improvement of Crocus sativus L. under tissue culture conditions. Plant Cell Tiss Org 121:325–334
- Patel P, Shah R, Joshi B, Ramar K, Natarajan A (2019) Molecular identification and biocontrol activity of sugarcane rhizosphere bacteria against red rot pathogen Colletotrichum falcatum. Biotechnol Rep 21:e00317
- Ramette A, Frapolli M, Défago G, Moënne-Loccoz Y (2003) Phylogeny of HCN synthase-encoding hcnBC genes in biocontrol fluorescent pseudomonads and its relationship with host plant species and HCN synthesis ability. Mol Plant Microbe Interact 16:525–535
- Rasul M, Yasmin S, Zubair M, Mahreen N, Yousaf S, Arif M, Sajid ZI, Mirza MS (2019) Phosphate solubilizers as antagonists for bacterial leaf blight with improved rice growth in phosphorus deficit soil. Biol Control 136:103997
- Rath M, Mitchell TR, Gold SE (2018) Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture-dependent. Microbiol Res 208:76–84
- Raza W, Ling N, Liu D, Wei Z, Huang Q, Shen Q (2016) Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol Res 192:103–113
- Renwick A, Campbell R, Coe S (1991) Assessment of in vivo screening systems for potential biocontrol agents of Gaeumannomyces graminis. Plant Pathol 40:524–532
- Rybakova D, Cernava T, Köberl M, Liebminger S, Etemadi M, Berg G (2016) Endophytes-assisted biocontrol: novel insights in ecology and the mode of action of Paenibacillus. Plant Soil 405:125–140
- Satyaprakash M, Nikitha T, Reddi EU, Sadhana B, Vani SS (2017) Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int J Curr Microbiol App Sci 6:2133–2144
- Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
- Senthil N, Raguchander T, Viswanathan R, Samiyappan R (2003) Talc formulated fluorescent pseudomonads for sugarcane red rot suppression and enhanced yield under field conditions. Sugar Tech 5:37–43
- Shoda M (2000) Bacterial control of plant diseases. J Biosci Bioeng 89:515–521
- Siddiqui S, Siddiqui ZA, Ahmad I (2005) Evaluation of fluorescent Pseudomonads and Bacillus isolates for the biocontrol of a wilt disease complex of pigeon pea. World J Microbiol Biotechnol 21:729–732
- Singh K, Singh RP (1989) Red rot. In: Ricaud CG, Egan BT, Gillaspie AG Jr, Hughes CG (eds) Sugarcane diseases of the world: major diseases. Elsevier, Amsterdam, pp 169–188
- Slama HB, Cherif-Silini H, ChenariBouket A, Qader M, Silini A, Yahiaoui B, Alenezi FN, Luptakova L, Triki MA, Vallat A, Oszako T (2019) Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against Fusarium. Front Microbiol 11:3236
- Solans M, Scervino JM, Messuti MI, Vobis G, Wall LG (2016) Potential biocontrol actinobacteria: rhizospheric isolates from the Argentine Pampas lowlands legumes. J Basic Microbiol 56:1289–1298
- Sulochana MB, Jayachandra SY, Kumar SKA, Dayanand A (2014) Antifungal attributes of siderophore produced by the Pseudomonas aeruginosa JAS-25. J Basic Microbiol 54:418–424
- Taechowisan T, Lu CH, Shen YM, Lumyong S (2005) Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology 151:1691–1695
- Tariq M, Yasmin S, Hafeez FY (2010) Biological control of potato black scurf by rhizosphere associated bacteria. Braz J Microbiol 41:439–451
- Velivelli SL, Kromann P, Lojan P, Rojas M, Franco J, Suarez JP, Prestwich BD (2015) Identification of mVOCs from Andean rhizobacteria and field evaluation of bacterial and mycorrhizal inoculants on growth of potato in its center of origin. Microb Ecol 69:652–667
- Velusamy P, Das J (2014) Identification and characterization of antifungal chitinase from Bacillus subtilis JD-09 and their role in inhibition of viable fungal growth. Int J Pharm Pharmeaut Sci 6:232–235
- Viswanathan R, Rajitha R, Sundar AR, Ramamoorthy V (2003) Isolation and identification of endophytic bacterial strains from sugarcane stalks and their in vitro antagonism against the red rot pathogen. Sugar Tech 5:25–29
- Viswanathan R, Samiyappan R (2001) Antifungal activity of chitinases produced by some fluorescent Pseudomonads against Colletotrichum falcatum Went causing red rot disease in sugarcane. Microbiol Res 155:309–314
- Xie S, Liu J, Gu S, Chen X, Jiang H, Ding T (2020) Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann Microbiol 70:1–10
- Xu SJ, Hong SJ, Choi W, Kim BS (2014) Antifungal activity of Paenibacillus kribbensis strain T-9 isolated from soils against several plant pathogenic fungi. Plant Pathol J 30:102
- Yoon GY, Lee YS, Lee SY, Park RD, Hyun HN, Nam Y, Kim KY (2012) Effects on Meloidogyne incognita of chitinase, glucanase and a secondary metabolite from Streptomyces cacaoi GY525. Nematology 14:175–184
- Zhao Y, Selvaraj JN, Xing F, Zhou L, Wang Y, Song H, Tan X, Sun L, Sangare L, Folly YM, Liu Y (2014) Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 9:1–11
- Cappuccino JC, Sherman N (1992) Microbiology. A laboratory manual, 3rd edn. Benjamin/Cummings Pub Co, New York, pp 125–179
- Hsu SC, Lockwood JL (1975) Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Environ Microbiol 29:422–426
- Özcan BD, Özcan N, Baylan M, Güzel AI (2013) Cloning and expression of β-1, 3-glucanase gene from Cellulosimicrobium cellulans in Escherichia coli DH5α. Kafkas Universitesi Veteriner Fakultesi Dergisi 19:523–528
Author Information
Department of Environmental Microbiology, Rhizosphere Biology Laboratory, School for Environmental Sciences, Babasaheb Bhimrao Ambedkar (A Central) University, Vidya Vihar, Lucknow, India