Laticifer development of seed-derived callus of Hevea brasiliensis Muell. Arg
*Article not assigned to an issue yet
Meksuwan Yurachat, Roongsattham Peerapat, Nualsri Charatsri, Meesawat Upatham
Research Articles | Published: 01 July, 2025
First Page: 0
Last Page: 0
Views: 719
Keywords: Callus, Growth curve, n Hevea brasiliensisn , Laticifer
Abstract
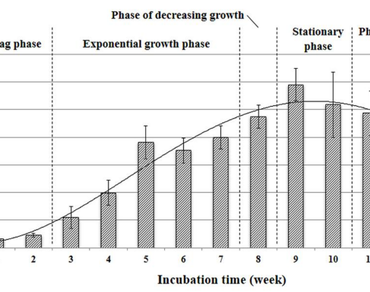
In vitro laticifer production during callus development was investigated in Hevea brasiliensis Muell. Arg. (RRIM600 cultivar). A seed-derived callus was grown for a month on a Murashige and Skoog medium supplemented with 2 mg/L of 2,4-dichlorophenoxyacetic acid, 2 mg/L of benzyladenine, and 5% sucrose before being transferred to the same medium with 3% sucrose for proliferation. For three months, the rising fresh weight of these calli was measured weekly in order to investigate their development. The callus generated from seeds followed a sigmoidal pattern, with stationary, exponential, and lag phases. In vitro laticifers were discovered after the second week of cultivation and reached their peak density in 8 weeks. After 12 weeks of culture, all laticifer types including those that had secreted latex, starch granule laticifers, and main laticifers were revealed. The findings revealed that the culture time interval influenced the in vitro proliferation of numerous laticifer types in the seed-derived callus of H. brasiliensis. During the two to eight weeks of cultivation, the density and breadth of laticifer cells in seed-derived callus rose slightly. A correlation between the culture period and the number of in vitro laticifers in seed-derived calli were also found.
References
Beck CB (2005) An introduction to plant structure and development. Cambridge, UK. Cambridge University Press
Biesboer DD, Mahlberg PG (1978) Laticifer starch grain morphology and laticifer evolution in Euphorbia (Euphorbiaceae). Nord J Bot 1(3):447–457. https://doi.org/10.1111/j.1756-1051.1981.tb00710.x
Bona CM, Santos GD, Biasi LA (2012) Lavandula calli induction, growth curve and cell suspension formation. Agrária 7(1):17–23. https://doi.org/10.5039/agraria.v7i1a1121
d’Auzac Jacob JL, Chrestin H (2000) Physiology of rubber tree latex. The laticiferous cell and latex a model of cytoplasm. CRC Press, Florida
Datta SK, De S (1986) Laticifer differentiation of Calotropis gigantean r.br. Ex ait. In cultures. Ann Bot 57:403–406
Demarco D, Castro MM, Ascensao L (2013) Two laticifer systems in Sapium haematospermum new records for Euphorbiaceae. Botany 91:545–554. https://doi.org/10.1139/cjb-2012-0277
Dhir SK, Shekhawat NS, Purohit SD, Arya HC (1984) Development of laticifers cells in callus cultures of Calotropis procera (Ait) R.BR. Plant Cell Rep 3:206–209. https://doi.org/10.1007/BF00270202
George EF, Hall MA, De Klerk GJ (2008) Plant propagation by tissue culture. 3rd edition, Vol. 1, Springer. Dordrecht, Netherlands
Gordana VN, Freshney RL (2006) Culture of cells for tissue engineering. John Wiley & Sons, Inc., Hoboken, New Jersey, Canada
Hagel JM, Yeung EC, Facchini PJ (2008) Got milk.the secret life of laticifers. Trends Plant Sci 13(12):631–639. https://doi.org/10.1016/j.tplants.2008.09.005
Hao BZ, Wu JL (2000) Laticifer differentiation in H. brasiliensis: induction by exogenous jasmonic acid and linolenic acid. Ann Bot 85:37–43. https://doi.org/10.1006/anbo.1999.0995
Keng CL, See KS, Hoon LP, Lim BP (2008) Effect of plant growth regulators and subculture frequency on callus culture and the establishment of Melastoma malabathricum cell suspension cultures for the production of pigments. Biotechnol 7(4):678–685. https://doi.org/10.3923/biotech.2008.678.685
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 5(3):473–497
Prakash MG, Gurumurthi K (2010) Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult 100:13–20. https://doi.org/10.1007/s11240-009-9611-1
Rajeswari B, Kumar SP, Rao PA, Kan PSSV (2014) A distribution and ultrastructure of laticifers in the phylloclade of Euphorbia caducifolia haines, a potential hydrocarbon yielding CAM plant. Am J Plant Sci 5:70–79. https://doi.org/10.4236/AJPS.2014.51011
Ramulifho E, Goche T, As JV, Tsilo TJ, Chivasa S, Ngara R (2019) Establishment and characterization of callus and cell suspension cultures of selected Sorghum bicolor (L.) Moench varieties: A resource for gene discovery in plant stress biology. Agronomy 9(218). https://doi.org/10.3390/agronomy9050218
Santos DN, Nunes CF, Soares JDR, Valente TCT, Alves E, Labory CRG, Pasqual M (2013) Cytological characterization of Jatropha curcas callus in different periods of cultivation. Crop Breed Appl Biot 13:228–233. https://doi.org/10.1590/S1984-70332013000400002
Smith RM (1992) Plant tissue culture. Techniques and experiments. Academic press. San Diego, CA, USA
Subroto T, Koningsveld GAV, Schreuder HA, Soedjanaatmadja UM, Beintema JJ (1996) Chitinase and beta-1,3-glucanase in the lutoid-body fraction of Hevea latex. Phytochemistry 43(1):29–37. https://doi.org/10.1016/0031-9422(96)00196-3
Tan D, Sun X, Zhang J (2011) Histochemical and immunohistochemical identification of laticifers cells in callus cultures derived from anthers of H. brasiliensis. Plant Cell Rep 30:1117–1124. https://doi.org/10.1007/s00299-011-1019-9
Tan D, Sun X, Zhang J (2014) Age-dependent and jasmonic acid-induced laticifer-cell differentiation in anther callus cultures of rubber tree. Planta 240(2):337–344. https://doi.org/10.1007/s00425-014-2086-2
Tan D, Kumpeangkeaw A, Sun X, Li W, Zhu Y, Zhang J (2019) Comparative morphology of in vivo and in vitro laticiferous cells and potential use of in vivo laticifers in early selection of rubber tree clones. Trees 33(1):193–203. https://doi.org/10.1007/s00468-018-1766-3
Thomas V, Premakumari D, Reghu CP, Panikkar AON, Saraswthy ACK (1995) Anatomical and histochemical aspects of bark regeneration in H. brasiliensis. Ann Bot 75:421–426. https://doi.org/10.1006/anbo.1995.1040
Venkatachalam P, Geetha N, Sangeetha P, Thulaseedharan A (2013) Natural rubber producing plants: an overview. AJB 12(12):1297–1310. https://doi.org/10.5897/AJBX12.016
Yodyotee Y, Nualsri C, Meesawat U (2018) Comparative anatomical study of in vivo and in vitro laticifers in two rubber tree (Hevea Brasiliensis muell. Arg.) cultivars (RRIM600 and RRIT402). SJPS 5(1):19–27
Yodyotee Y, Roongsattham P, Nualsri C, Meesawat U (2016) In vitro laticifer identification in young shoot-derived callus of H. brasiliensis. WJST 14(7):563–570
Author Information
Faculty of Technology and Environment, Prince of Songkla University, Phuket, Thailand