Leaf development and anatomy of in vitro-grown Polygala paniculata L. are affected by light quality, gelling agents, and sucrose
Nery Lays Araújo, Batista Diego Silva, Rocha Diego Ismael, Felipe Sérgio Heitor Sousa, Queiroz Matheus da Costa, Silva Priscila Oliveira, Ventrella Marília Contin, Otoni Wagner Campos, Queiroz Matheus da Costa, Silva Priscila Oliveira
Research Articles | Published: 02 February, 2021
First Page: 19
Last Page: 28
Views: 4020
Keywords: Agar, Leaf anatomy, Light quality, Micropropagation, Phytagel®
Abstract
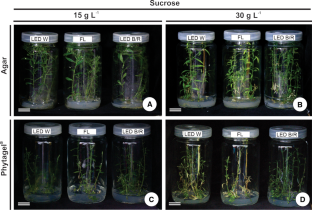
Plant in vitro growth performance is influenced by environmental, physical, and chemical conditions. The present study evaluated the mutual effect of light, gelling agents, and sucrose concentration on leaf anatomical structure of in vitro-grown Polygala paniculata. Nodal segments were inoculated on agar- or Phytagel®-gelified medium supplemented with 15 g L−1 or 30 g L−1 sucrose. Cultures were grown under fluorescent lamps, light-emitting diodes (LEDs) with white light, and LEDs with blue and red light at 56 µmol m−2 s−1 photon flux. After 45 days of culture, leaf histology was assessed. The leaves of P. paniculata had an uniseriate epidermis and dorsiventral mesophyll. Gelling agents had antagonistic effects in relation to sucrose concentration. Growth on agar and 30 g L−1 sucrose induced greater leaf blade thickness, with expanded palisade and spongy parenchyma but looser intercellular spaces. In contrast, growth on Phytagel® led to significantly thicker palisade parenchyma at 15 g L−1 sucrose. Fluorescent lamp light favored in vitro leaf development of P. paniculata grown on agar; whereas spectral quality had no effect on leaf blade thickness in Phytagel®-grown plants. In summary, mutual combinations of gelling agent, light quality, and sucrose concentration influence the establishment and anatomic structure of leaves in P. paniculata plantlets grown in vitro. This result will facilitate studies on in vitro propagation of this important medicinal species.
References
- Alvarenga ICA, Pacheco FV, Silva ST, Bertolucci SKV, Pinto JEBP (2015) In vitro culture of Achillea millefolium L.: quality and intensity of light on growth and production of volatiles. Plant Cell Tissue Organ Cult 122:299–308
- Alvarez C, Saéz P, Sáez K, Sánchez-Olate M, Ríos D (2012) Effects of light and ventilation on physiological parameters during in vitro acclimatization of Gevuina avellana mol. Plant Cell Tissue Organ Cult 110:93–101
- Arena C, Tsonev T, Doneva DV, De Micco M, Michelozzi C, Brunetti M, Centritto S, Fineschi V, Velikova F (2016) The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ Exp Bot 16:8472
- Ayuso M, García-Pérez P, Ramil-Rego P, Gallego PP, Barreal ME (2019) In vitro culture of the endangered plant Eryngium viviparum as dual strategy for its ex situ conservation and source of bioactive compounds. Plant Cell Tissue Organ Cult 138:427–435
- Barbosa LMP, Paiva Neto VB, Dias LLC, Festucci-Buselli RA, Alexandre RS, Iarema L, Finger FL, Otoni WC (2013) Análises bioquímicas e morfo-anatômicas em vitroplantas de morangueiro hiper-hídricas afetadas pelo BA e agentes geleificantes. Rev Ceres 60:2
- Batista DS, Castro KM, Silva AR, Teixeira ML, Sales TA, Soares LI, Cardoso MG, Santos MO, Viccini LF, Otoni WC (2016) Light quality affects in vitro growth and essential oil profile in Lippia alba (Verbenaceae). In Vitro Cell Dev Biol Plant 52:276–282
- Batista DS, Castro KM, Koehler AD, Porto BN, Silva AR, Souza VC, Teixeira ML, Cardoso MG, Santos MO, Viccini LF, Otoni WC (2017a) Elevated CO2 improves growth, modifies anatomy, and modulates essential oil qualitative production and gene expression in Lippia alba (Verbenaceae). Plant Cell Tissue Organ Cult 128:357–368
- Batista DS, Dias LLC, Rêgo MM, Saldanha CW, Otoni WC (2017b) Flask sealing on in vitro seed germination and morphogenesis of two types of ornamental pepper explants. Cienc Rural 47:3
- Batista DS, Felipe SHS, Silva TD, de Castro KM, Mamedes-Rodrigues TC, Miranda NA, Ríos-RíosAM, Faria DV, Fortini EA, Chagas K, Torres-Silva G, Xavier A, Arencibia AD, Otoni WC (2018) Light quality in plant tissue culture: does it matter? In Vitro Cell Dev Biol Plant 54:195–215
- Bhattacharya P, Dey S, Bhattacharyya C (1994) Use of low-cost gelling agents and support matrices for industrial scale plant tissue culture. Plant Cell Tissue Organ Cult 37:15–23
- Cavallaro V, Patane C, Cosentino SL, Di Silvestro I, Copani V (2014) Optimizing in vitro large scale production of giant reed (Arundo donax L.) by liquid medium culture. Biomass Bioenergy 69:21–27
- Cruz CD (2013) GENES—a software package for analysis in experimental statistics and quantitative genetics. Acta Sci Agron 35:271–227
- Debergh PC (1983) Effects of agar brand and concentration on the tissue culture medium. Physiol Plant 59:270–276
- Dobránszki J, Magyar-Tábori K, Tombácz E (2011) Comparison of the rheological and diffusion properties of some gelling agents and blends and their effects on shoot multiplication. Plant Biotechnol Rep 5:345–352
- Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248:1–18
- Faria DV, Correia LNF, Souza MVC, Ríos-Ríos AM, Vital CE, Batista DS, Costa MGC, Otoni WC (2019) Irradiance and light quality affect two annatto (Bixa orellana L.) cultivars with contrasting bixin production. J Photochem Photobiol B 197:111549
- Franck T, Crèvecoeur M, Wuest J, Greppin H, Gaspar T (1997) Cytological comparison of leaves and stems of Prunus avium L. shoots cultured on a solid medium with agar or gelrite. Biotech Histochem 73:32–43
- Gupta SD, Jatothu B (2013) Fundamentals and applications of light emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol Rep 7:211–220
- Gupta SD, Karmakar A (2017) Machine vision based evaluation of impact of light emitting diodes (LEDs) on shoot regeneration and the effect of spectral quality on phenolic content and antioxidant capacity in Swertia chirata. J Photochem Photobiol B 174:162–172
- Gyula P, Schäfer E, Nagy F (2003) Light perception and signalling in higher plants. Curr Opin Plan Biol 6:446–452
- Hsie BS, Bueno IS, Bertolucci SKV, Carvalho AA, Cunha SHB, Martins ER, Pinto JEBP (2019) Study of the influence of wavelengths and intensities of LEDs on the growth, photosynthetic pigment, and volatile compounds production of Lippia rotundifolia Cham in vitro. J Photochem Photobiol B 198:111577
- Isah T, Umar S (2020) Influencing in vitro clonal propagation of Chonemorpha fragrans (moon) Alston by culture media strength, plant growth regulators, carbon source and photo periodic incubation. J For Res 31:27–43
- Ivanova M, Van Staden J (2011) Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla. Plant Cell Tissue Organ Cult 104:13–21
- Jain N, Babbar SB (2002) Gum katira—a cheap gelling agent for plant tissue culture media. Plant Cell Tissue Organ Cult 71:223–229
- Karnovsky MJ (1965) A formaldehyde–glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
- Kim SJ, Hahn EJ, Heo JW, Paek KY (2004) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of Chrysanthemum plantlets in vitro. Sci Hortic 101:143–151
- Kozai T (2010) Photoautotrophic micropropagation—environmental control for promoting photosynthesis. Prop Ornam Plants 10:188–204
- Lapa FR, Gadoti VM, Missau FC, Pizzolatti MG, Marques MC, Dafré AL, Farina M, Rodrigues ALS, Santos ARS (2009) Antinociceptive properties of the hydroalcoholic extract and the flavonoid rutin obtained from Polygala paniculata L. in mice. Basic Clin Pharmacol 104:306–315
- Lapa FR, Soares KC, Rattmanna YD, Crestiana S, Missaub FC, Pizzolatti MG, Marques MCA, Rieck L, Santos ARS (2011) Vasorelaxant and hypotensive effects of the extract and the isolated flavonoid rutin obtained from Polygala paniculata L. J Pharm Pharmacol 63:875–881
- Li H, Tang C, Xu Z (2013) The effects of different light qualities on rapeseed (Brassica napus L.) plant growth and morphogenesis in vitro. Sci Hortic 150:117–124
- Li CX, Xu ZG, Dong RQ, Chang SX, Wang LZ, Khalil URM, Tao JM (2017) An RNA-seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red LED light, and white fluorescent light. Front Plant Sci 8:1–16
- Lorenzi H, Matos FJA (2002) Plantas Medicinais do Brasil: nativas e exóticas. Nova Odessa, São Paulo: Instituto Plantarum. p 386
- Mamedes-Rodrigues TC, Batista DS, Napoleão TA, Cruz ACF, Fortini EA, Nogueira FTS, Romanel E, Otoni WC (2018) Lignin and cellulose synthesis and antioxidative defense mechanisms are affected by light quality in Brachypodium distachyon. Plant Cell Tissue Organ Cult 133:1–14
- Martins JPR, Pasqual M, Martins AD, Ribeira SF (2015) Effects of salts and sucrose concentrations on in vitro propagation of Billbergia zebrina (Herbert) Lindley (Bromeliaceae). Aust J Crop Sci 9:85–91
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
- Nogueira FLP, Fernandes SOB, Reis GM, Matheus ME, Fernandes PD, Lage CLS, Menezes FS (2005) Atividade analgésica e antiedematogênica de Polygala paniculata L. (Polygalaceae) selvagem e obtida por micropropagação. Rev Bras Farmacogn 154:310–315
- O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
- Paiva Neto VB, Otoni WC (2003) Review—Carbon sources and their osmotic potential in plant tissue culture: does it matter? Sci Hortic 97:193–202
- Persson C (2001) Phylogenetic relationships in Polygalaceae based on plastid DNA sequences from the trnL-F region. Taxon 50:763–779
- Phillips GC, Garda M (2019) Plant tissue culture media and practices: an overview. In Vitro Cell Dev Biol Plant 55:242–257
- Pizzolatti MG, Mendes BG, Soldi C, Missau FC, Bortoluzzi JH, Carasek E (2011) Analysis of volatile compounds released from flowers and roots of Polygala cyparissias and Polygala paniculata by Headspace/SPME. J Essent Oil Res 21:255–258
- Pruski K, Kozai T, Lewis T, Astatkie T, Nowak J (2000) Sucrose and light effects on in vitro cultures of potato, chokecherry and saskatoon berry during low temperature storage. Plant Cell Tissue Organ Cult 63:215–221
- Puchooa D, Purseramen PN, Rujbally BR (1999) Effects of medium support and gelling agent in the tissue culture of tobacco (Nicotiana tabacum). Sci Technol 3:129–145
- Reis LB, Costa RR, Otoni WC (2005) Influência de agentes gelificantes na organogênese in vitro de explantes juvenis de maracujazeiro-amarelo (Passiflora edulis f. flavicarpa Degener). Plant Cell Cult Microprop 1:80–88
- Rodrigues FA, Rezende RAL, Pasqual M, Lopes MTG (2017) Solidifying agents and activated charcoal for in vitro culture of Solanum sessiliflorum. Pesq Agropec Bras 52:1123–1126
- Sæbø A, Krekling T, Appelgren M (1995) Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult 41:177–185
- Saldanha CW, Otoni CG, Notini MM, Kuki KN, Cruz ACF, Rubio Neto A, Dias LLC, Otoni WC (2013) A CO2-enriched atmosphere improves in vitro growth of brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. In Vitro Cell Dev Biol Plant 49:433–444
- Saldanha CW, Otoni CG, Rocha DI, Cavatte PC, Detmann KSC, Tanaka FAO, Dias LL, DaMatta FM, Otoni WC (2014) CO2-enriched atmosphere and supporting material impact the growth, morphophysiology and ultrastructure of in vitro. Plant Cell Tissue Organ Cult 118:87–99
- Schuerger AC, Brown C, Stryjewski EC (1997) Anatomical features of pepper plants (Capsicum annuum L.) growth under red light emitting diodes supplemented with blue or far-red light. Ann Bot 79:273–282
- Serret MD, Trillas MI (2000) Effects of light and sucrose levels on the anatomy, ultrastructure, and photosynthesis of gardenia Jasminoides ellis leaflets cultured in vitro. Int J Plant Sci 161:281–289
- Shin KS, Murthy HN, Heo JW, Hahn EJ, Yoeup K (2008) The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol Plant 30:339–343
- Silva MMA, Oliveira ALB, Oliveira-Filho RA, Camara T, Willadino L, Gouveia-Neto A (2016) The effect of spectral light quality on in vitro culture of sugarcane. Acta Sci 38:157–161
- Silva TD, Chagas K, Batista DS, Felipe SHS, Louback E, Machado LT, Fernandes AM, Buttrós VHT, Koehler AD, Farias LM, Santos AF, Silva PO, Otoni WC (2019) Morphophysiological in vitro performance of Brazilian ginseng (Pfaffia glomerata (Spreng.) Pedersen) based on culture medium formulations. In Vitro Cell Dev Biol Plant 55:454–467
- Silva TD, Batista DS, Fortini EA, Castro KM, Felipe SHS, Fernandes AM, Sousa RMJ, Chagas K, Silva JVS, Correia LNF, Farias LM, Leite JPV, Rocha DI, Otoni WC (2020) Blue and red light affects morphogenesis and 20-hydroxyecdisone content of in vitro Pfaffia glomerata accessions. J Photochem Photobiol B 203:111761
- Smith H (1982) Light quality, photo perception, and plant strategy. Ann Rev Plant Physiol 33:481–518
- Stuefer JF, Huber H (1998) Differential effects of light quantity and spectral light quality on growth, morphology and development of two stoloniferous Potentilla species. Oecologia 117:1–8
- Szopa A, Ekiert H (2016) The importance of applied light quality on the production of lignans and phenolic acids in Schisandra chinensis (Turcz.) Baill. cultures in vitro. Plant Cell Tissue Organ Cult 127:115–121
- Victório VC, Carriço JB, Lage CLS (2011) Polygala paniculata: a source of methyl salicylate produced through plant tissue culture. Rev Ceres 58:269–272
- Vieira LN, Fraga HPF, Anjos KG, Puttkammer CC, Scherer RF, Silva DA, Guerra MP (2015) Light-emitting diodes (LED) increase the stomata formation and chlorophyll content in Musa acuminata (AAA) ‘Nanicão Corupá’ in vitro plantlets. Theor Exp Plant Physiol 27:91–98
- Zhou Y, Yan J, Xu BY, Wang BC (2019) The study on mechanical properties of Phytagel medium. IOP Conf Ser Earth Environ Sci 346:012089
Author Information
Instituto Federal do Norte de Minas Gerais-IFNMG, Almenara, Brazil