Media optimization using Box Behnken design for enhanced production of biomass, beta-carotene and lipid from Dunaliella salina
Research Articles | Published: 13 November, 2019
First Page: 31
Last Page: 39
Views: 4118
Keywords: Beta-carotene, Lipids, Box Behnken design, Response surface methodology
Abstract
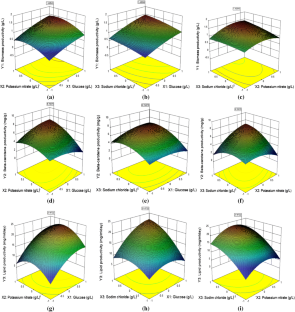
Dunaliella salina a halotolerant microalga is well known for a high fatty acid and beta-carotene content, which makes it a potent source at a commercial level. The current study focuses on optimizing commercially known media using Box Behnken design to attain higher yields of biomass, beta-carotene, and lipids simultaneously. The optimal medium conditions as per response surface methodology were glucose, potassium nitrate, sodium chloride at a concentration of 13.23 g/L, 3.145 g/L and 35.6 g/L, respectively while maintaining the concentration of other nutrients unchanged. Maximum yield of biomass, beta-carotene and lipid productivity attained experimentally using the optimized media was 1.24 g/L, 6.07 mg/g and 20.7 mg/L/day, respectively than their original values i.e., 0.571 g/L of biomass, 4.18 mg/g of beta-carotene and 13.2 mg/L/day of lipid content. Biomass yield was increased by 2.17 folds, beta-carotene and lipid were increased by 1.45 folds and 1.56 folds, respectively.
References
- Ahmed RA, He M, Aftab RA, Zheng S, Nagi M, Bakri R, Wang C (2017) Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci Rep 7(1):8118
- Azma M, Mohamed MS, Mohamad R, Rahim RA, Ariff AB (2011) Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochem Eng J 53(2):187–195
- Bonnefond H, Moelants N, Talec A, Mayzaud P, Bernard O, Sciandra A (2017) Coupling and uncoupling of triglyceride and beta-carotene production by Dunaliella salina under nitrogen limitation and starvation. Biotechnol Biofuels 10:25
- Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
- Chen H, Jiang JG, Wu GH (2009) Effects of salinity changes on the growth of Dunaliella salina and its isozyme activities of glycerol-3-phosphate dehydrogenase. J Agric Food Chem 57(14):6178–6182
- Chen M, Tang H, Ma H, Holland TC, Ng KY, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102(2):1649–1655
- Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
- Del Campo JA, García-González M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
- Dominguez Teles I (2016) The fatter the better: selecting microalgae cells for outdoor lipid production. Wageningen University. https://library.wur.nl/WebQuery/wurpubs/508992
- Fakhry EM, El Maghraby DM (2015) Lipid accumulation in response to nitrogen limitation and variation of temperature in Nannochloropsis salina. Botanical studies 56(1):6
- Fazeli MR, Tofighi H, Samadi N, Jamalifar H, Fazeli A (2006) Carotenoids accumulation by Dunaliella tertiolecta (Lake Urmia isolate) and Dunaliella salina (ccap 19/18 & wt) under stress conditions. DARU J Pharm Sci 14(3):146–150
- García-González M, Moreno J, Manzano JC, Florencio FJ, Guerrero MG (2005) Production of Dunaliella salina biomass rich in 9-cis-beta-carotene and lutein in a closed tubular photobioreactor. J Biotechnol 115(1):81–90
- Gary H, Masao O (2001) Impact of algal research in aquaculture. J Phycol 37:968–974
- Gómez PI, Barriga A, Cifuentes AS, González MA (2003) Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) Chlorophyta. Biol Res 36(2):185–192
- Hallenbeck PC, Grogger M, Mraz M, Veverka D (2015) The use of Design of Experiments and Response Surface Methodology to optimize biomass and lipid production by the oleaginous marine green alga, Nannochloropsis gaditana in response to light intensity, inoculum size and CO2. Bioresour Technol 184:161–168
- Hosseini Tafreshi A, Shariati M (2009) Dunaliella biotechnology: methods and applications. J Appl Microbiol 107(1):14–35
- Kirrolia A, Bishnoi NR, Singh R (2014) Response surface methodology as a decision-making tool for optimization of culture conditions of green microalgae Chlorella spp. for biodiesel production. Ann Microbiol 64(3):1133–1147
- Lamers PP, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2008) Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol 26:631–638
- Lamers PP, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2012) Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J Biotechnol 162(1):21–27
- Mata-Gómez LC, Montañez JC, Méndez-Zavala A, Aguilar CN (2014) Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Fact 13:12
- Mojaat M, Pruvost J, Foucault A, Legrand J (2008) Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem Eng J 39(1):177–184
- Morowvat MH, Younes G (2016) Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal Agric Biotechnol 7:217–223
- Oren A (2014) The ecology of Dunaliella in high-salt environments. J Biol Res 21(1):23
- Rabbani S, Beyer P, Lintig J, Hugueney P, Kleinig H (1998) Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol 116(4):1239–1248
- Singh G, Jawed A, Paul D, Bandyopadhyay KK, Kumari A, Haque S (2016) Concomitant production of lipids and carotenoids in Rhodosporidium toruloides under osmotic stress using response surface methodology. Front Microbiol 7:1686
- Skorupskaite V, Makareviciene V, Levisauskas D (2015) Optimization of mixotrophic cultivation of microalgae Chlorella sp. for biofuel production using response surface methodology. Algal Res 7:45–50
- Smith RT, Bangert K, Wilkinson SJ, Gilmour DJ (2016) Synergistic carbon metabolism in a fast growing mixotrophic freshwater microalgal species Micractinium inermum. Biomass Bioenerg 2015(82):73–86
- Srinivasan R, Kumar VA, Kumar D, Ramesh N, Babu S, Gothandam KM (2015) Effect of dissolved inorganic carbon on β-carotene and fatty acid production in Dunaliella sp. Appl Biochem Biotechnol 175(6):2895–2906
- Sun XM, Ren LJ, Zhao QY, Ji XJ, Huang H (2018) Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol Biofuels 11:272
- Wang J, Yang H, Wang F (2014) Mixotrophic cultivation of microalgae for biodiesel production: status and prospects. Appl Biochem Biotech 172:3307–3329
- Wu Z, Duangmanee P, Zhao P, Ma C (2016) The effects of light, temperature, and nutrition on growth and pigment accumulation of three Dunaliella salina strains isolated from saline soil. Jundishapur J Microbiol 9(1):1–9
Author Information
Plant Biotechnology Laboratory, Department of Biotechnology, Delhi Technological University, New Delhi, India