Molecular characterization of late embryogenesis abundant proteins encoding genes from wild sorghum genotype IS-18,909 expressed under drought stress
Ujjainkar N. R., Panpatil A. U., Chimote V. P., Pawar B. D., Kale A. A.
Research Articles | Published: 18 March, 2024
First Page: 911
Last Page: 920
Views: 3136
Keywords: Drought stress, Hydrophilic proteins, Conserved motifs, LEA1, LEA3
Abstract
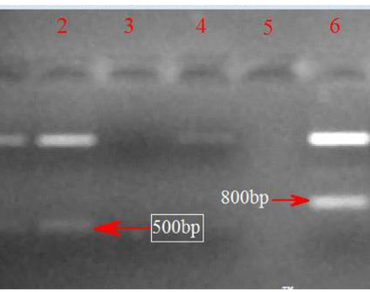
As a drought-tolerant crop, sorghum is an ideal plant for identifying genes conferring drought tolerance. In the present study, LEA1 and LEA 3 genes expressing in response to PEG-6000 induced osmotic stress were isolated from drought and heat tolerant wild sorghum genotype IS-18,909. Gene-specific primers were designed and used for cDNA synthesis of late embryogenesis abundant (LEA) proteins encoding LEA1 (547 bp) and LEA3 (817 bp) genes. Sequence analysis of LEA1 (547 bp) and LEA3 (817 bp) cDNA confirmed the presence of corresponding full-length coding sequences. LEA1 and LEA3 proteins had specific 20 nucleotides and 11 nucleotides consensus sequences, respectively, and other conserved sequences. These drought-induced proteins are extremely hydrophilic, resistant to dehydration, and high in amino acid residues with the sulfhydryl group (serine, threonine). They are composed largely of the amino acids glycine, alanine, and glutamine and lack cysteine and tryptophan. The genes conferring drought tolerance may provide a foundation for improving sorghum productivity under water deficit conditions.
References
Abdel-Ghany SE, Ullah F, Ben-Hur A, Reddy AS (2020) Transcriptome analysis of drought-resistant and drought-sensitive sorghum (Sorghum bicolor) genotypes in response to PEG-induced drought stress. Int J Mol Sci 21(3):772. https://doi.org/10.3390/ijms21030772
Abdul Aziz M, Sabeem M, Mullath SK, Brini F, Masmoudi K (2021) Plant Group II LEA proteins: intrinsically disordered structure for multiple functions in response to environmental stresses. Biomolecules 11(11):1662. https://doi.org/10.3390/biom11111662
Ali MA, Jabran K, Awan SI, Abbas A, Zulkiffal M, Acet T, Farooq J, Rehman A (2011) Morpho-physiological diversity and its implications for improving drought tolerance in grain sorghum at different growth stages. Aust J Crop Sci 5:311
Amara I, Zaidi I, Masmoudi K, Ludevid MD, Pagès M, Goday A, Brini F (2014) Insights into late embryogenesis abundant (LEA) proteins in plants: from structure to the functions. Am J Plant Sci 5(22):3440–3455. https://doi.org/10.4236/ajps.2014.522360
Badigannavar A, Teme N, de Oliveira AC, Li G, Vaksmann M, Viana VE, Ganapathi TR, Sarsu F (2018) Physiological, genetic and molecular basis of drought resilience in sorghum [Sorghum bicolor (L.) Moench]. Ind J Plant Physiol 23:670–688. https://doi.org/10.1007/s40502-018-0416-2
Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148(1):6. https://doi.org/10.1104/pp.108.120725
Bienert S, Waterhouse A, de Beer TA, Tauriello G, Studer G, Bordoli L, Schwede T (2017) The SWISS-MODEL repository - new features and functionality. Nucleic Acids Res 45(D1):D313. https://doi.org/10.1093/nar/gkw1132
Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant 97(4):795. https://doi.org/10.1111/j.1399-3054.1996.tb00546.x
Cuming A (1999) In: Casey R, Shewry PR, Proteins S (eds) LEA proteins. Kluwer Academic, Dordrecht, pp 753–780. https://doi.org/10.1007/978-94-011-4431-5_32
Damame SV, Naik RM, Chimote VP, Munjal SV (2016) Molecular analysis of rabi sorghum genotypes differing in osmolytes accumulation under water stress. Int J Bio-Resour Stress Manag 7(5):11207. https://doi.org/10.23910/IJBSM/2016.7.5.1544
Dure III, L (2001) Occurrence of a repeating 11-mer amino acid sequence motif in diverse organisms. Protein Pept Let 8(2):115. https://doi.org/10.2174/0929866013409643
Fracasso A, Trindade LM, Amaducci S (2016) Drought stress tolerance strategies revealed by RNA-Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol 16(1):1. https://doi.org/10.1186/s12870-016-0800-x
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein Identification and Analysis Tools on the ExPASy server. In: Walker JM (ed) The Proteomics protocols Handbook. Springer protocols handbooks. Humana. https://doi.org/10.1385/1-59259-890-0:571
Guex N, Peitsch MC, Schwede T (2009) Automated comparative protein structure modeling with SWISS-MODEL and Swiss‐PdbViewer: a historical perspective. Electrophoresis 30(S1):S162. https://doi.org/10.1002/elps.200900140
Hunault G, Jaspard E (2010) LEAPdb: a database for the late embryogenesis abundant proteins. BMC Genom 11(1):1–9. https://doi.org/10.1186/1471-2164-11-221
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. https://doi.org/10.1038/nprot.2015.053
Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40(D1):D302–D305. https://doi.org/10.1093/nar/gkr931
Li X, Cao J (2016) Late Embryogenesis Abundant (LEA) gene family in maize: identification, evolution, and expression profiles. Plant Mol Biol Rep 34:15–28. https://doi.org/10.1007/s11105-015-0901-y
Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH (2017) CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45(D1):D200–D203. https://doi.org/10.1093/nar/gkw1129
McCormick RF, Truong SK, Sreedasyam A, Jenkins J, Shu S, Sims D, Kennedy M, Amirebrahimi M, Weers BD, McKinley B, Mattison A (2018) The Sorghum bicolor reference genome: improved assembly, gene annotations, a transcriptome atlas, and signatures of genome organization. Plant J 93(2):338. https://doi.org/10.1111/tpj.13781
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51(5):914–916. https://doi.org/10.1104/pp.51.5.914
Minh BM, Linh NT, Hanh HH, Hien LT, Thang NX, Hai NV, Hue HT (2019) A LEA gene from a Vietnamese maize landrace can enhance the drought tolerance of transgenic maize and tobacco. Agronomy 9(2):62. https://doi.org/10.3390/agronomy9020062
Nagaraju M, Kumar SA, Reddy PS, Kumar A, Rao DM, Kavi Kishor PB (2019) Genome-scale identification, classification, and tissue specific expression analysis of late embryogenesis abundant (LEA) genes under abiotic stress conditions in Sorghum bicolor L. PLoS ONE 14(1):e0209980. https://doi.org/10.1371/journal.pone.0209980
Ogbaga CC, Stepien P, Dyson BC, Rattray NJW, Ellis DI, Goodacre R (2016) Biochemical analyses of sorghum varieties reveal differential responses to drought. PLoS ONE 11(5):e0154423. https://doi.org/10.1371/journal.pone.0154423
Oliver MJ, Farrant JM, Hilhorst HW, Mundree S, Williams B, Bewley JD (2020) Desiccation tolerance: avoiding cellular damage during drying and rehydration. Annu Rev Plant Biol 29(71):435–460. https://doi.org/10.1146/annurev-arplant-071219-105542
Ongom PO, Volenec JJ, Ejeta G (2016) Selection for drought tolerance in sorghum using desiccants to simulate post-anthesis drought stress. Field Crops Res 198:312–321. https://doi.org/10.1016/j.fcr.2016.03.015
Pardo J, Wai CM, Chay H, Madden CF, Hilhorst HW, Farrant JM, VanBuren R (2020) Intertwined signatures of desiccation and drought tolerance in grasses. Proc Natl Acad Sci USA 117(18):10079–10088. https://doi.org/10.1073/pnas.2001928117
Pedrosa AM, Martins CDPS, Gonçalves LP, Costa MGC (2015) Late embryogenesis abundant (LEA) constitutes a large and diverse family of proteins involved in development and abiotic stress responses in sweet orange (Citrus sinensis L. Osb). PLoS ONE 10(12):pe0145785. https://doi.org/10.1371/journal.pone.0145785
Sanchez AC, Subudhi PK, Rosenow DT, Nguyen HT (2002) Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Mol Biol 48:713–726. https://doi.org/10.1023/A:1014894130270
Sigrist CJ, Cerutti L, de Castro E, Langendijk-Genevaux PS, Bulliard V, Bairoch A, Hulo N (2010) PROSITE, a protein domain database for functional characterization and annotation. Nucleic Acids Res 38:D161–D166. https://doi.org/10.1093/nar/gkp885
Sweet RM, Eisenberg D (1983) Correlation of sequence hydrophobicities measures similarity in three-dimensional protein structure. J Mol Biol 171(4):479–488. https://doi.org/10.1016/0022-2836(83)90041-4
Author Information
State Level Biotechnology Centre, Mahatma Phule Krishi Vidyapeeth, Rahuri, Ahmednagar, India