Monitoring of catechin content by high performance liquid chromatography during the development of seed and pods of Saraca asoca (Roxb.) De Willd
Dahiya Jyoti, Kumar Deepak, Bolleddu Rajesh, Dutta Sreya, Mall Simmi, Hazra Kalyan, Mangal Anupam K.
Research Articles | Published: 28 March, 2023
First Page: 341
Last Page: 354
Views: 3733
Keywords: n Saracan asocan , Catechin, HPLC, TLC-fingerprint, DPPH-TLC
Abstract
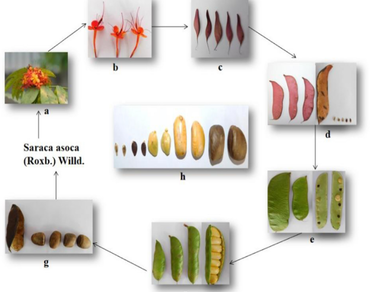
Saraca asoca (Roxb.) Willd, is an indigenous medicinal plant of family Caesalpiniaceae. Different parts of this plant including seeds and pods, possess numerous biological activities on account of the presence of various phytoconstituents. Phenolic compounds, especially catechin, is the critical constituent responsible for the many pharmacological activity of Saraca asoca. The level of phytoconstituent in plant part depends upon the harvesting time. The bio-active contents changes during the development process of plant parts and is affected by many factors. Hence, the present study aims to find the best harvest time for pods and seeds by monitoring the changes in morphological, organoleptic and microscopic characters, followed by physiochemical analysis and chromatographic studies during development process. The chromatographic techniques like thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC) were used to develop the fingerprint profile and determination of catechin content, respectively. The catechin content was also determined in stem bark to find any chance of substitution with seed and pod. The antioxidant potential was also determined by DPPH (2,2-diphenyl-1-picrylhydrazyl)-TLC method. It was found that seed (SD-4) and pod (PD-1) contained the maximum catechin content. This study provides the basis for collecting seed and pod at the best harvesting time. Also, the comparative studies for catechin content between stem bark, seed and pods suggest the use of seed and pods in place of stem bark but only at the proper age, as the excessive usage of stem bark may decrease the plants life expectancy by damaging the plant system.
References
Alaerts G et al (2012) Similarity analyses of chromatographic fingerprints as tools for identification and quality control of green tea. J Chromatogr B 910:1–10. https://doi.org/10.1016/j.jchromb.2012.04.031
Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (basel) 6(42):1–23. https://doi.org/10.3390/plants6040042
Anonymous (2004) The ayurvedic pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare, Department of Ayurveda, Yoga and Naturpathy, Unani, sidha and Homeopathy (AYUSH), New Delhi; Part I, Volume IV:152–74.
Anonymous (2011) The ayurvedic pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare, Department of Ayurveda, Yoga and Naturpathy, Unani, sidha and Homeopathy (AYUSH), New Delhi; Part I, Volume VIII: 193–195
Begum SN, Ravikumar K, Ashok VDK (2014) Asoka—an important medicinal plant, its market scenario and conservation measures in India. Curr Sci 107:26–28
Bhalerao S, Verma D, Didwana VK, Teli N (2014) Saraca asoca (Roxb.), De Wild: an overview. Ann Plant Sci 3(07):770–775
Ciesla L, Staszek D, Kowalska T, Waksmundzka-Hajnos M (2013) The Use of TLC-DPPH test with image processing to study direct antioxidant activity of phenolic acid fractions of selected lamiaceae family species. J AOAC Int 96(6):1228–1232. https://doi.org/10.5740/jaoacint.SGECiesla
Dahiya J, Singh J, Kumar A, Sharma A (2016) Isolation, characterization and quantification of an anxiolytic constituent—mahanimbine, from Murraya koenigii Linn. Spreng Leaves J Ethnopharmacol 193:706–711. https://doi.org/10.1016/j.jep.2016.10.014
Dahiya J, Mangal AK, Bolleddu R, Dutta S, Kharwar S, Hazra K, Prasad PVV (2022) Enrichment of hydroalcoholic extract of Trigonella foenum-graecum L. seed with major components using TLC fingerprint, image analysis, and design of experiment studies. J Drug Res. in Ayurvedic Sci 7(2):133–144. https://doi.org/10.4103/jdras.jdras_20_22
Dhanani T, Singh R, Kumar S (2017) Extraction optimization of gallic acid, (+)-catechin, procyanidin-B2, (-)-epicatechin, (-)-epigallocatechin gallate, and (-)-epicatechin gallate: their simultaneous identification and quantification in Saraca asoca. J Food Drug Anal 25(3):691–698. https://doi.org/10.1016/j.jfda.2016.08.004
Ghosh S, Majumder M, Ganguly NK, Chatterjee BP (1999) Saracin: A lectin from Saraca indica seed integument induces apoptosis in human T-lymphocytes. Arch Biochem Biophys 371(2):163–168. https://doi.org/10.1006/abbi.1999.1418
Goodarzi M, Russell PJ, Heyden YV (2013) Similarity analyses of chromatographic herbal fingerprints: a review. Anal Chim Acta 804:16–28. https://doi.org/10.1016/j.aca.2013.09.017
Hegde S, Hegde HV, Jalalpure SS, Peram MR, Pai SR, Roy S (2017) Resolving identification issues of Saraca asoca from its adulterant and commercial samples using phytochemical markers. Pharmacogn Mag 13(2):266–272. https://doi.org/10.4103/pm.pm_417_16
ICH (1994) Harmonised Tripartite Guideline. Validation of analytical procedures: text and methodology Q2(R1).
Isemura M (2019) Catechin in human health and disease. Molecules 24(3):528. https://doi.org/10.3390/molecules24030528
Ketkar P, Nayak S, Pai S, Joshi RK (2015) Determining seasonal changes in three major phenolic compounds from bark of Saraca asoca in comparison with local market sample using RP-HPLC analysis. Natl Acad Sci Lett 38:403–407. https://doi.org/10.1007/s40009-015-0382-4
Kulkarni KS, Patil LJ, Khanvilkar V, Kadam VJ (2014) Fingerprinting techniques in herbal standardization. Indo Am J Pharm Res 4(02):1049–1062 (Corpus ID: 26915949)
Li S, Han Q, Qiao C, Song J, Lung Cheng C, Xu H (2008) Chemical markers for the quality control of herbal medicines: an overview. Chin Med 3(7):1–16. https://doi.org/10.1186/1749-8546-3-7
Lohar D (2007) Protocol for testing of ayurvedic, siddha and unani medicines. 1st ed. Ghaziabad: Govt. of India, Department of AYUSH, Ministry of Health and Family Welfare, Pharmacopoeial Laboratory for Indian Medicine.
Martiely S et al (2012) Harvest time and plant age on the content and chemical composition of the essential oil of Alpinia zerumbet. Hortic Bras 30:385–390. https://doi.org/10.1590/S0102-05362012000300005
Momin RK, Kadam VB (2011) Determination of ash values of some medicinal plants of genus sesbania of marathwada region in maharashtra. J Phytology 3:2 (Corpus ID: 86188353)
Nicoletti M (2011) HPTLC fingerprint: a modern approach for the analytical determination of Botanicals. Braz J Pharmacogn 21(5):818–823. https://doi.org/10.1590/S0102-695X2011005000131
Olapade AA, Ajai OA, Ajai IA (2014) Physical and chemical properties of Cassia sieberiana seeds. Int Food Res J 21(2):767–772
Olasoji JO, Aluko AO, Adeniyan ON, Olanipekun SO, Olosunde AA, Okoh JO (2012) Effect of time of harvest on physiological maturity and kenaf (Hibiscus canabinus) seed quality. Afr J Plant Sci 6(10):282–289. https://doi.org/10.5897/AJPS12.083
Özcan MM, Juhaimi FA, Gülcü M, Uslu N, Geçgel Ü, Ghafoor K, Dursun N (2017) Effect of harvest time on physico-chemical properties and bioactive compounds of pulp and seeds of grape varieties. J Food Sci Technol 54(8):2230–2240. https://doi.org/10.1007/s13197-017-2658-9
Pawar SS, Jadhav MG (2015) Determination of extractive value and phytochemical analysis of Bacopa monnieri (L). State level seminar on recent advances in life sciences
Pradhan P et al (2009) Saraca Asoca (Ashoka): a review. J Chem Pharm Res 1(1):62–71
Ranade AV, Acharya R (2015) Influence of time factor on phytoconstituents in certain ayurvedic medicinal plant: a comprehensive review. J Pharma Sci Inn 4:235–241. https://doi.org/10.7897/2277-4572.04553
Rathee P, Rathee S, Rathee D, Rathee D (2010) Quantitative estimation of (+)-Catechin in stem bark of Saraca asoca using HPTLC. Der Pharma Chemica 2(1):306–314
Rezaei F, VanderGheynst JS (2010) Critical moisture content for microbial growth in dried food-processing residues. J Sci Food Agric 90(12):2000–2005. https://doi.org/10.1002/jsfa.4044
Ristivojevic PM, Morlock GE (2016) The influence of pre-processing method on multivariate image analysis in high performance thin layer chromatography fingerprinting. JPC-Modern TLC 29:310–317. https://doi.org/10.1556/1006.2016.29.4.10
Ristivojevic P et al (2014) Pattern recognition methods and multivariate image analysis in HPTLC fingerprinting of propolis extracts. J Chemom 28:301–310. https://doi.org/10.1002/cem.2592
Saha J, Mukherjee S, Gupta K, Gupta B (2013) High-performance thin-layer chromatographic analysis of antioxidants present in different parts of Saraca asoca (Roxb.) de Wilde. J Pharm Res 7(9):798–803. https://doi.org/10.1016/j.jopr.2013.10.004
Sahu D, Bolleddu R, Das M, Das D, Mandal TK, Debnath SK, Barik LD, Dahiya J, Mandal S, Prasad PVV (2022) Pharmacognostical and phytochemical studies of leaves of Plectranthus amboinicus (Lour.) Spreng (Parnayavani). Res J Pharm Tech 15(2):717–722. https://doi.org/10.52711/0974-360x2022.00119
Sehwag S, Das M (2013) Antioxidant activity: an overview. J Food Sci Technol 2:1–10
Shawky E, Kheir R (2018) Rapid discrimination of different Apiaceae species based on HPTLC fingerprints and targeted flavonoids determination using multivariate image analysis. Phytochem Anal 29(5):452–462. https://doi.org/10.1002/pca.2749
Shirolkar A, Gahlaut A, Chhillar A, Dabur R (2013) Quantitative analysis of catechins in Saraca asoca and correlation with antimicrobial activity. J Pharm Anal 3(6):421–428. https://doi.org/10.1016/j.jpha.2013.01.007
Singh KK, Mridula D, Barnwal P, Rehal J (2012) Physical and chemical properties of flaxseed. Int Agrophys 26(4):423–426. https://doi.org/10.2478/v10247-012-0060-4
Singh S et al (2015) Phytomedicinal importance of Saracaasoca (Ashoka): an exciting past, an emerging present and a promising future. Curr Sci 109:1790–1801. https://doi.org/10.18520/CS/V109/I10/1790-1801
Smitha GR, Thondaiman V (2016) Reproductive biology and breeding system of Saraca asoca (Roxb.) De Wilde: a vulnerable medicinal plant. Springerplus 5(1):20–25. https://doi.org/10.1186/s40064-016-3709-9
Srivastava GN, Bagchi GD, Srivastava AK (2008) Pharmacognosy of Ashoka stem bark and its adulterants. Pharm Bio 26:65–72. https://doi.org/10.3109/13880208809053892
Tailor CS, Goyal A (2014) Antioxidant activity by dpph radical scavenging method of ageratum conyzoides Linn. leaves. A J Ethnomed 1:244–249 (Corpus ID: 83681273)
Tamprasit K, Weerapreeyakul N, Sutthanut K, Thukhammee W, Wattanathorn J (2019) Harvest age effect on phytochemical content of white and black glutinous rice cultivars. Molecules 24(24):4432. https://doi.org/10.3390/molecules24244432
Tewari R et al (2017) Extraction, quantification and antioxidant activities of flavonoids, polyphenols and pinitol from wild and cultivated Saraca asoca bark using RP-HPLC-PDA-RI method. Ind Crops Prod 103:73–80. https://doi.org/10.1016/j.indcrop.2017.03.036
Wojcieszak D, Przybył J, Czajkowski Ł, Majka J, Pawłowski A (2022) Effects of harvest maturity on the chemical and energetic properties of corn stover biomass combustion. Materials 15(8):2831. https://doi.org/10.3390/ma15082831
Author Information
Department of Pharmacognosy, Central Ayurveda Research Institute, CCRAS, Ministry of AYUSH, Kolkata, India