Non-embryogenic synthetic seed production of Lawsonia inermis L. and HPTLC analysis of lawsone
Research Articles | Published: 14 September, 2024
First Page: 2250
Last Page: 2261
Views: 1833
Keywords: In vitro node, High performance Thin layer chromatography (HPTLC), In vivo node, Lawsone, Synthetic seed
Abstract
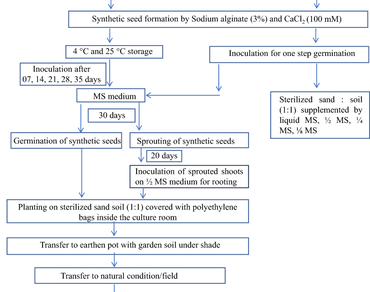
An attempt was made to standardize a protocol for synthetic seed production and subsequent plant regeneration of Lawsonia inermis by calcium alginate encapsulation of both in vivo and in vitro regenerated axenic nodal segments. The combination of 3% sodium alginate and 100 mM CaCl2 was found as the optimum encapsulation matrix showing the highest sprouting (73.3%), and germination rate (66.6%) for synthetic seed derived from in vivo nodal explant and highest sprouting (80%), and germination rate (70%) for synthetic seeds derived from in vitro nodal explants on Murashige and Skoog’s (MS) basal medium. It was confirmed that the synthetic seeds could be stored at 4 ºC up to two weeks without losing much of their germination potential i.e., about 56.6% and 66.6% germination was recorded for in vivo and in vitro nodal explants encapsulated synthetic seeds respectively. Lawsone is the most important compound present in L. inermis. Thus, the biochemical fidelity of the plants derived from synthetic seeds (encapsulated axenic in vitro nodal explant) vis-a-vis the mother plant was confirmed by checking the lawsone through HPTLC. This standardized protocol for synthetic seed production of L. inermis could be utilized for the exchange and transfer of germplasm to distant places or laboratories as well as for its propagation. At the same time, this protocol of synthetic seed production could be useful for the production of biochemically identical and stable plants of L. inermis, in terms of lawsone, for pharmaceutical and cosmetic industries.
References
Ahmad N, ANIS M (2010) Direct plant regeneration from encapsulated nodal segments of Vitex negundo. Biol Plant 54:748–752
Batiha GE, Teibo JO, Shaheen HM, Babalola BA, Teibo TKA, Al-Kuraishy HM, Al-Garbeeb AI, Alexiou A, Papadakis M (2023) Therapeutic potential of Lawsonia inermis Linn: a comprehensive overview. Naunyn-Schmiedeberg’s Arch Pharmacol 394(6):3525–3540
Behera S, Rout KK, Panda PC, Naik SK (2020) Production of non-embryogenic synthetic seeds for propagation and germplasm transfer of Hedychium coronarium J Koenig. J Appl Res Med Aromat Plants 19:100271
Behera B, Behera S, Shasmita MD, Barik DP, Naik SK (2021) Regeneration of plants from alginate-encapsulated axenic nodal segments of Paederia foetida L. – A medicinally important and vulnerable plant species. J Plant Biotechnol 48:255–263
Chaudhary GD, Goyal S, Poonia P (2010) Lawsonia inermis Linnaeus: a phytopharmacological review. Int J Pharma Sci Drug Res 2:91–98
Chauhan R, Banjare P, Parey SK, Anjum A, Quraishi A (2024) Low-temperature storage in dark condition improved the in vitro regeneration of Plumbago zeylanica synthetic seeds: a medicinally valuable species. In Vitro Cell Dev Biol -Plant. https://doi.org/10.1007/s11627-024-10416-1
Danso KE, Ford-Lloyd BV (2003) Encapsulation of nodal cuttings and shoot tips for storage and exchange of cassava germplasm. Plant Cell Rep 21:718–725
Faisal M, Alatar AA, Ahmad N, Anis M, Hegazy AK (2012) Assessment of genetic fidelity in Rauvolfia serpentina plantlets grown from synthetic (encapsulated) seeds following in vitro storage at 4 ◦C. Molecules 17:5050–5061
Gantait VSJ, Majee A (2017) Artificial Seed Production of Tylophora indica for interim storing and swapping of germplasm. Hortic Plant J 3:41–46
Jyotshna PG, Singh DK, Luqman S, Shanker K (2017) Validated method for quality assessment of henna (Lawsonia inermis L.) leaves after postharvest blanching and its cosmetic application. Ind Crops Prod 95:33–42
Kumar S, Rai MK, Singh N, Mangal M (2010) Alginate-encapsulation of shoot tips of jojoba [Simmondsia chinensis (Link) Schneider] for germplasm exchange and distribution. Physiol Mol Biol Plants 16:379–382
Larkin P, Davies PA, Tanner GJ (1988) Nurse culture of low numbers of Medicago and Nicotiana protoplasts using calcium alginate beads. Plant Sci 58:203–210
Mikhaeil BR, Badria FA, Maatooq GT, Amer MMA (2004) Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch C J Biosci 59:468–476
Moharana A, Das A, Subudhi E, Naik SK, Barik DP (2017) High frequency shoot proliferation from cotyledonary node of Lawsonia inermis L. and validation of their molecular finger printing. J Crop Sci Biotech 20:405–416
Moharana A, Das A, Subudhi E, Naik SK, Barik DP (2018a) Assessment of genetic fidelity using random amplified polymorphic DNA and inter simple sequence repeats markers of Lawsonia inermis L. plants regenerated by axillary shoot proliferation. Proc Natl Acad Sci India Sect B Biol Sci 88:133–141
Moharana A, Barik DP, Naik SK, Rout KK (2018b) Comparative thin-layer chromatographic studies and development of a high-performance thin-layer chromatography method for the quantification of lawsone in natural and micropropagated plant parts of Lawsonia inermis L. J Planar Chromat 31:155–162
Naik SK, Chand PK (2006) Nutrient-alginate encapsulation of in vitro nodal segments of pomegranate (Punica granatum L.) for germplasm distribution and exchange. Sci Hortic 108:247–252
Naz R, Anis M, Alatar AA, Ahmad A, Naaz A (2018) Nutrient alginate encapsulation of nodal segments of Althaea officinalis L. for short – term conservation and germplasm exchange. Plant Biosyst 152:1256–1262
Prasad A, Singh M, Yadav NP, Mathur AK, Mathur Molecular A (2014) chemical and biological stability of plants derived from artificial seeds of Centella asiatica (L.) Urban—an industrially important medicinal herb. Ind Crops Prod 60:205–211
Qahtan AA, Alatar AA, Faisal M (2023) In vitro regeneration, phytochemical profiling and antioxidant activity in Ruta chalepensis plants established from alginate encapsulated synthetic seeds. South Afr J Botany 161:575–585
Rai MK, Jaiswal VS, Jaiswal U (2009) Effect of selected amino acids and polyethylene glycol on maturation and germination of somatic embryos of guava (Psidium guajava L.) Sci Hortic. Physiol Mol Biol Plants 121:233–236
Ram K, Shekhawat NS (2011) Micropropagation of commercially cultivated Henna (Lawsonia inermis) using nodal explants. Physiol Mol Biol Plant 17:281–289
Rathore MS, Kheni J (2017) Alginate encapsulation and in vitro plantlet regeneration in critically endangered medicinal plant, Withania coagulans (Stocks) Dunal. Proc Natl Acad Sci India Sect B Biol Sci 87:129–134
Redenbaugh K, Slade DT, Viss PR, Fujii JA (1987) Encapsulation of somatic embryos in synthetic seed coats. Hortic Sci 22:803–809
Remya M, Bai Narmatha V, Mutharaian VN (2013) In vitro regeneration of Aristolochia tagala and production of artificial seeds. Biol Plant 57:210–218
Rout GR, Das G, Samantaray S, Das P (2001) In vitro micropropagation of Lawsonia inermis (Lythraceae). Rev Biol Trop 49:957–963
Semwal RB, Semwal DK, Combrinck S, Cartwright-Jones C, Viljoen A (2014) “Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J Ethnopharmacol 155(1):80–103
Sharma S, Shahzad A, Teixeira JA, S (2013) Synseed technology-a complete synthesis. Biotechnol Adv 31:186–207
Singh AK, Sharma M, Varshney R, Agarwal SS, Bansal KC (2006) Plant regeneration from alginate-encapsulated shoot tips of Phyllanthus amarus Schum and Thonn, a medicinally important plant species. In Vitro Cell Dev Biol –– Plant 42:109–113
Singh P, Jain K, Jain B, Khare S (2012) In vitro micropropagation of Lawsonia inermis: an important medicinal plant. Int J Curr Res Rev 22:29–34
Singh DK, Luqman S, Mathur AK (2015) “Lawsonia inermis L. – A commercially important primaeval dying and medicinal plant with diverse pharmacological activity: A review. Ind Crops Prod 65:269–286
Ved DK, Goraya GS (2007) Demand and supply of medicinal plants in India. NMPB, New Delhi & FRLHT, Bangalore, India 18(85):210–252
Verma SK, Rai MK, Asthana P, Jaiswal VS, Jaiswal U (2010) In vitro plantlets from alginate-encapsulated shoot tips of Solanum nigrum L. Sci Hortic 124:517–521
DataHorizzon Research, (https://datahorizzonresearch.com/henna-powder-market-3047; last assessed: 11.05.2024)
Author Information
Department of Botany, Ravenshaw University, Cuttack, India