Phenolic profile, antioxidant and antibiofilm properties of Valeriana alliariifolia Vahl
*Article not assigned to an issue yet
Research Articles | Published: 21 November, 2025
First Page: 0
Last Page: 0
Views: 486
Keywords: n Valeriana alliariifolia Vahl, Antioxidants, LC–MS/MS, Phenolics, Biofilm inhibition, Climatic adaptation
Abstract
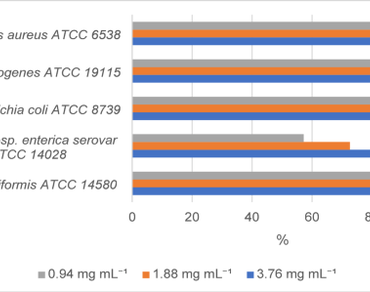
Valeriana alliariifolia Vahl has long been valued in traditional medicine for its sedative and antispasmodic effects. Nevertheless, compared with other members of the Valeriana genus, this species has rarely been examined in detail. In this study, we focused on how environmental stress—particularly the cold conditions typical of continental climates—affects its phenolic composition, antioxidant defenses, and antibiofilm activity. Exploring these aspects helps reveal how V. alliariifolia adapts biochemically to harsh environments, adding a new perspective to the understanding of its potential pharmacological value. Methanolic extracts obtained from leaf and rhizome tissues were analyzed for total phenolic content and detailed phenolic composition. Antioxidant capacity was evaluated through total antioxidant capacity (TAC) and enzymatic markers, including superoxide dismutase, catalase (CAT), and glutathione (GSH). The antibiofilm effects of each extract were tested against five pathogenic bacterial strains using the crystal violet microplate assay. Extracts from rhizomes showed significantly elevated TAC, CAT, and GSH levels compared to those from leaves (p < 0.05). LC–MS/MS analysis revealed a distinct distribution of phenolics across tissues; rhizomes were particularly rich in flavonoids such as kaempferol and quercetin, whereas leaves contained higher levels of phenolic acids, including fumaric acid and protocatechuic acid. Both extracts demonstrated significant antibiofilm activity, particularly against Bacillus licheniformis, Escherichia coli, and Listeria monocytogenes. Valeriana alliariifolia Vahl seems to adapt to the stresses of a cold continental environment by adjusting its antioxidant enzyme systems and phenolic metabolism. This pioneering report underscores the species potential to combat biofilms, a significant finding that could inspire future uses in these areas. The results highlight its promise as a natural source for antioxidant and antibiofilm applications, offering hope for practical applications in the future.
References
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63:3523–3543
Bardakci H, Demirci B, Yesilada E, Kirmizibekmez H, Baser KHC (2012) Chemical composition of the essential oil of the subterranean parts of. Rec Nat Prod 6:89–92
Bhatt ID, Dauthal P, Rawat S, Gaira KS, Jugran A, Rawal RS, Dhar U (2012) Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Jones. Sci Hortic 136:61–68
Chaieb K, Kouidhi B, Jrah H, Mahdouani K, Bakhrouf A (2011) Antibacterial activity of Thymoquinone, an active principle of and its potency to prevent bacterial biofilm formation. BMC Complem Altern Med 11:29
Chaves De Jesus P, Rego Rodrigues Silva DM, Macedo Moura PH, Gopalsamy RG, Dias Silva EE, Dos Santos Barreto M, Santana Santos R, Santos Martins AY, De Freitas Almeida AG, Santana Corrêa AK, Alves Da Mota Santana L, Hariharan G, Gibara Guimarães A Pinto Borges L (2025) The in vitro pharmacokinetics of medicinal plants: a review. Pharmaceuticals 18:551
Cruz-Carrion A, Calani L, De Azua MJR, Mena P, Del Rio D, Suarez M, Arola-Arnal A (2022) (Poly)phenolic composition of tomatoes from different growing locations and their absorption in rats: a comparative study. Food Chem 388:132984
Çelik C, Kirmizibekmez H (2025) The genus L.: ethnopharmacology, phytochemistry and biological activities-an updated review. Phytochem Rev. https://doi.org/10.1007/s11101-024-10061-x
Davis PH (1972) Flora of Turkey and the East Aegean Islands. University Press, Edinburg
Düzgüner V, Erbil N (2019) Ardahan’da Yetişen Kediotunun (Valeriana officinalis L.) Antimikrobiyal Ve Antioksidan Potansiyelinin Belirlenmesi. Türk Tarım Ve Doğa Bilimleri Dergisi 6(2):271–275. https://doi.org/10.30910/turkjans.557113
Howitz KT, Sinclair DA (2008) Xenohormesis: sensing the chemical cues of other species. Cell 133:387–391
İçigen HÖ (2019) Bitki doku kültürü yöntemi ile elde edilen bazı bitki türlerinin antimikrobiyal aktivitelerinin belirlenmesi. Fen Bilimleri Enstitüsü.
Karatoprak G (2025) Valeriana alliariifolia Adams Kök Ekstresinin SH-SY5Y ve PC-12 Hücre Hatları Üzerindeki Toksik Etkilerinin Değerlendirilmesi ve Antioksidan Aktivitesinin Araştırılması. J Inst Sci Technol 15:83–91
Jugran AK, Bahukhandi A, Dhyani P, Bhatt ID, Rawal RS, Nandi SK (2016) Impact of altitudes and habitats on Valerenic Acid, Total Phenolics, Flavonoids, Tannins, and Antioxidant Activity of Valeriana jatamansi. Appl Biochem Biotechnol 179:911–926
Kapoor D, Singh S, Kumar V, Romero R, Prasad R, Singh J (2019) Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 19:100182
Li JC, Li XL, Wang CF, Zhang ML, Wang YMH, QH, (2022) The potential of as a traditional Chinese medicine: traditional clinical applications, bioactivities, and phytochemistry. Front Pharmacol 13:973138
Lister E, Wilson P (2001) Measurement of total phenolics and ABTS assay for antioxidant activity (personal communication), vol 7. Crop Research Institute, Lincoln, pp 235–239
Liu HY, Prentice EL, Webber MA (2024) Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob Resist 2:27
Liu XM, Hu YT, Xue ZY, Zhang X, Liu XF, Liu GW, Wen MZ, Chen AJ, Huang B, Li XA, Yang N, Wang J (2023) Valtrate, an iridoid compound in, elicits anti-glioblastoma activity through inhibition of the PDGFRA/MEK/ERK signaling pathway. J Transl Med 21:147
Mohammadi Z, Pishkar L, Eftekhari Z, Barzin G, Babaeekhou L (2024) Evaluation of the antimicrobial and cytotoxic activity of cultivated Valeriana officinalis. Plant Sci Today 11:145–155
Nagahama N, Gastaldi B, Clifford MN, Manifesto MM, Fortunato RH (2021) The influence of environmental variations on the phenolic compound profiles and antioxidant activity of two medicinal Patagonian valerians (Valeriana carnosa Sm. and V. clarionifolia Phil.). AIMS Agric Food 6:106–124
Nandhini S, Narayanan KB, Ilango K (2018) Valeriana officinalis: a review of its traditional uses, phytochemistry and pharmacology. Asian J Pharm Clin Res 11:36–41
Olszowy M (2019) What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol Biochem 144:135–143
Qaderi MM, Martel AB, Strugnell CA (2023) Environmental factors regulate plant secondary metabolites. Plants 12:447
Qian Z, He L Li F (2024). Understanding cold stress response mechanisms in plants: an overview. Frontiers in Plant Science, Volume 15 - 2024.
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sen-Utsukarci B, Taskin T, Goger F, Tabanca N, Estep AS, Kessler SM, Akbal-Dagistan O, Bardakci H, Kurkcuoglu M, Becnel J, Kiemer A, Mat A (2019) Chemical composition and antioxidant, cytotoxic, and insecticidal potential of in Turkey. Arh Hig Rada Toksikol-Arch Ind Hyg Toxicol 70:207–218
Taherpour AA, Maroofi H, Bajelani O, Larijani K (2010) Chemical composition of the essential oil of Valeriana alliariifolia Adams of Iran. Nat Prod Res 24:973–978
Tamfu AN, Ceylan O, Fru GC, Ozturk M, Duru ME, Shaheen F (2020) Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of Persoon. Microbial Pathog 144:104191
Uçar E, Atas M, Çilesiz Y, Çinbilgel I, Eruygur N, Karaköy T (2021) Variation of bioactivities and phytochemical compositions of Valeriana dioscoridis Sm. extracts. Ksu Tarim Ve Doga Dergisi-Ksu Journal of Agriculture and Nature 24:738–746
Yüceol F (2021). Türkiye Valerıana L.(Caprıfolıaceae) Cinsinin Taksonomik Revizyonu.
Zuo GL, Kim HY, Quispe YNG, Wang ZQ, Kim KH, Arce PGH, Lim SS (2021) Ruiz & Pav. root extract: a new source of caffeoylquinic acids with antioxidant and aldose reductase inhibitory activities. Foods 10:1079
Author Information
Faculty of Health Sciences, Ardahan University, Ardahan, Turkey