Phenolics compounds, evaluation of Alpha-amylase, alpha‐glucosidase inhibitory capacity and antioxidant effect from Globularia alypum L
Research Articles | Published: 06 April, 2021
First Page: 477
Last Page: 484
Views: 8493
Keywords: Globularia alypum , Phenolics compounds, Oxidative stress, Diabetes mellitus, RP-HPLC-PDA
Abstract
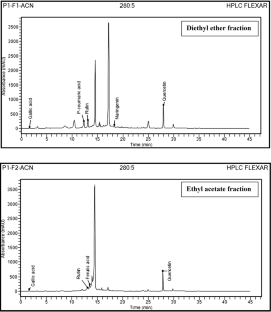
The aim of this study was to evaluate in vitro antioxidant and inhibitory Alpha-amylase and Alpha-glucosidase activities of fractions from Globularia alypum. Phenolic and flavonoids contents were estimated by Folin–Ciocalteu and aluminum chloride colorimetric methods, respectively. Identification of phenolic constituents was carried out by RP-HPLC–PDA method for diethyl ether and ethyl acetate fractions. Antioxidant activity was assessed by DPPH, FRAP and antihemolysis assays. Antidiabetic activity was evaluated as the first report on in vitro inhibitory effect of fractions on key diabetes enzymes: Alpha-amylase and Alpha-glucosidase. Fractions from G. alypum revealed high enzyme inhibitory activities and potent antioxidant effect. They showed significant antihemolytic results (HT50 from 194 ± 4.86 to 289 ± 5.67 min). Diethyl ether fraction presented the highest amounts in phenolic (331.88 ± 17.80 µg GAE/mg DF) and flavonoids (223.46 ± 2.37 µg CE/mg DF). HPLC analysis revealed the presence of gallic acid, p-coumaric acid, rutin, naringenin and quercetin. It exhibited the highest antioxidant activity assayed by DPPH (IC50 = 20.54 ± 0.48 µg/ml) and FRAP (EC50 = 283.7 ± 82.59 µg/ml). It showed the best inhibitory effect on Alpha-amylase (IC50 = 0.57 ± 0.05 mg/ml) and Alpha-glucosidase (IC50 = 0.52 ± 0.02 mg/ml). Globularia alypum is rich in phenolics and flavonoids. It is a promising source of potent natural antioxidants. It is suggested as an effective plant material for antidiabetic drugs.
References
- Alqahtani AS, Hidayathulla S, Rehman MT, ElGamal AA, Al-Massarani S, Razmovski-Naumovski V et al (2020) Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 10:61
- Ardestani A, Yazdanparast R (2007) Inhibitory effects of athl acetate extract of Teucriumpolium in vitro protein glycolxydation. Food Chem Toxicol 45:2402–2411
- Asraoui F, Kounnoun A, El Cadi H, Cacciola F, Oulad El Majdoub Y, Alibrando F, Mandolfino F, Dugo P, Mondello L, Louajri A (2021) Phytochemical Investigation and Antioxidant Activity of Globularia alypum L. Molecules 26:759
- Bahadori MB, Zengin G, Bahador iS, Dinparast L, Movahhedin N (2018) Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int J Food Prop 21(1):198–208
- Belkhiri F, Baghiani A, Zerroug MM, Arrar L (2017) Investigation of antihemolytic, xanthine oxidase inhibition, antioxidant and antimicrobial properties of Salvia verbenaca L. aerial part extract. Afr J Tradit Complement Altern Med 14:273–281
- Boumerfeg S, Baghiani A, Messaoudi D, Khennouf S, Arrar L (2009) Antioxidant properties and xanthine oxidase inhibitory effects of Tamus communis L. root extracts. Phytother Res 23:283–288
- Bouriche H, Kada S, Senator A, demirtas I, Ozen T, Toptanci B, Kizil G, Kizil M (2017) Phenolic content and biomolecule oxidation protective activity of Globularia alypum extracts. Braz Arch Biol Technol 60:e17160409
- Boussoualim N, Krache I, Baghiani A, Trabsa H, Aouachria S, Arrar L (2016) Human Xanthine oxidase inhibitory effect, antioxidant in vivo of Algerian extracts (Globularia alypum L.). Int J Pharmacogn Phytochem Res 8:645–650
- Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352
- Giuberti G, Rocchetti G, Lucini L (2020) Interactions between phenolic compounds, amylolytic enzymes and starch: an updated overview. Curr Opin Food Sci 31:102–113
- IDF: International Diabetes Federation 2019. The 9th edition of IDF Diabetes Atlas
- Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL et al (2015) Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes 8:396
- Jasicka-Mislak I, Poliwoda A, Petecka M, Buslovych O, Shlyapnikov VA, Wieczorek PP (2018) Antioxidant phenolic compounds in Salvia officinalis L. and Salvia sclarea L. Ecol Chem Eng S 25:133–142
- Kailash C, Pradeep S, Shridhar D, Jain SK (2019) Diabetes Mellitus and oxidative stress: a co-relative and therapeutic approach. J Clin Diagn Res 13(5):7–12
- Kasangana P, Eid H, Nachar A, Stevanovic T, Haddad PS (2019) Further isolation and identification of anti-diabetic principles from root bark of Myrianthus arboreus P. Beauv.: the ethyl acetate fraction contains bioactive phenolic compounds that improve liver cell glucose homeostasis. J Ethnopharmacol 245(1):112167
- Kraza L, Senoussi MM, Halis Y (2020) In vitro investigation of the antioxidant and antimicrobial effects of hydro-alcoholic and aqueous extracts of Globularia alypum L. Acta Scientifica Naturalis 7:46–58
- Lankatillake C, Huynh T, Dias DA (2019) Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 15:105
- Li H-B, Cheng K-W, Wong C-C, Fan K-W, Chen F, Jiang Y (2007) Evaluation of antioxidant capacity and total phenolic content of different fraction of selected microalgae. Food Chem 102:771–776
- Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395:203–230
- Maritim AC, Sanders RA, Watkins JB (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
- Markham KR (1982) Technics of flavonoids identification. Academic Press, London, p 113
- Miara MD, Bendif H, Rebbas K, Rabah B, Hammou MA, Maggi F (2019) Medicinal plants and their traditional uses in the highland region of Bordj Bou Arreridj (Northeast Algeria). J Herb Med 16:100262
- Niki E, Komuro E, Takahashi M, Urano S, Ito E, Terao K (1988) Oxidative hemolysis of erythrocytes and its inhibition by free radical scavengers. J Biol Chem 263:19809–19814
- Oyaizu M (1986) Studies on products of browning reaction Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet 44(6):307–315
- Pradeep KP, Jen-MingY, Yen-Hsiang C, Wei-Wen S (2019) Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int J Biol Macromol 136:661–667
- Suwalsky M, Duguet J, Speisky H (2017) An in vitro study of the antioxidant and antihemolytic properties of Buddleja globosa (Matico). J Membr Biol 250:239–248
- Telli A, Esnault M-A, Khelil OElH, A (2016) An ethnopharmacological survey of plants used in traditional diabetes treatment in south-eastern Algeria (Ouargla province). J Arid Environ 127:82–92
- Thalapaneni N, Chidambaram K, Ellappan T, Sabapathi M, Mandal S (2008) Inhibition of carbohydrate digestive enzymes by Talinium portulacifolium (Forssk) Leaf extracts. J Complem Integr Med 5:11
- Thirumurugan K, Jaykumar AB, Hyder MSSH (2011) Screening of Fifeen Indian ayurvedic plants for alpha-glucosidase inhibitory activity and enzyme kinetics. Int J Pharm Sci 3:267–274
- Tiss M, Souiy Z, Achour L, Hamden K (2020) Anti-obesity, anti-hyperglycaemic, anti-antipyretic and analgesic activities of Globularia alypum extracts. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2020.1773865
- Venkatesan T, Choi Y-W, Kim Y-K (2019) Impact of different extraction solvents on phenolic content and antioxidant potential of pinus densiflora bark extract. Hindawi Bio Med Res Int. doi.https://doi.org/10.1155/2019/3520675
Author Information
Laboratoire Antibiotiques Antifongiques : Physico-chimie, Synthèse et Activité Biologique, Faculté des sciences de la nature et de la vie et des sciences de la terre et de l’univers, Université de Tlemcen, Tlemcen, Algérie