Phytochemical profile, antioxidant and antibacterial activities of the ethanolic rice (Oryza sativa) leaf extract
Research Articles | Published: 27 December, 2024
First Page: 2384
Last Page: 2391
Views: 2582
Keywords: Antimicrobial, Antioxidant, Gas chromatography–mass spectrometry, n Oryza sativan , Rice leaf
Abstract
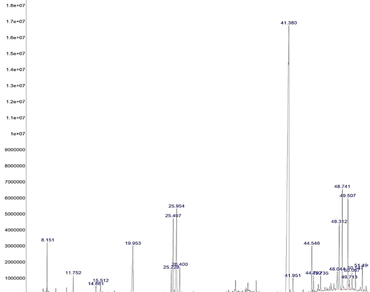
Recent studies have demonstrated the nutritional values and pharmacological properties of rice leaves at young development stages. This study aimed to examine the rice leaf extract aged three weeks for phytochemical profile, antioxidant and antibacterial activities. The result showed that the total phenolic, total flavonoid and total chlorophyll contents of rice leaves accounted for 91.94 ± 1.43 mg GAE/g, 65.02 ± 0.54 mg QE/g, and 26.2 ± 0.33 mg/g dried weight, respectively. Rice leaf extract showed values of IC50 in three assays, 2,2-diphenyl-1-picrylhydrazyl (354.98 ± 4.11 µg/mL), 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (808.97 ± 6.45 µg/mL) and reducing power (493.25 ± 5.96 µg/mL). It was active against Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli, ranging between 5.33 and 21.33 mm, 3.67–19.33 mm and 4.80–20.24 mm in terms of inhibition zone, respectively. GC-MS profiling of rice leaves elucidated a total of 22 active compounds, mainly summarized in fatty acid esters, steroids, terpenoids, phthalate esters, and fatty amides, which were reported to possess different biological activities. Our findings indicated the antioxidant and antibacterial properties of rice leaves. Rice leaves at young development stages could be used as a biomaterial for food and nutraceutical applications.
References
Alonso A-M, Reyes-Maldonado OK, Puebla-Pérez AM et al (2022) GC/MS analysis, antioxidant activity, and antimicrobial effect of Pelargonium peltatum (Geraniaceae). Molecules 27:3436. https://doi.org/10.3390/molecules27113436
Al-Rajhi AMH, Mashraqi A, Al Abboud MA et al (2022) Screening of bioactive compounds from endophytic marine-derived fungi in Saudi Arabia: antimicrobial and anticancer potential. Life 12:1182. https://doi.org/10.3390/life12081182
Anjum F, Touqeer S, Khan MY et al (2024) Pharmacognostic Evaluation, Chemical Characterization, and Antibacterial Activity of Bassia indica (Wight) A.J. Scott. Plants 13:1753. https://doi.org/10.3390/plants13131753
Baghernezhad N, Archangi B, Savari A, Amini F (2024) Molecular Docking of Secondary Metabolites of Marine Macroalgae Sargassum vulgare Against Exotoxin A. Plant Algae Environ 8:1327–1336. https://doi.org/10.48308/jpr.2024.235599.1078
Baloyi IT, Adeosun IJ, Bonvicini F, Cosa S (2023) Biofilm reduction, in-vitro cytotoxicity and computational drug-likeness of selected phytochemicals to combat multidrug-resistant bacteria. Sci Afr 21:e01814. https://doi.org/10.1016/j.sciaf.2023.e01814
Berwal M, Haldhar S, Ram C et al (2021) Effect of extraction solvent on total phenolics, flavonoids and antioxidant capacity of flower bud and foliage of Calligonum polygonoides L. Indian J Agric Biochem 34:61–67. https://doi.org/10.5958/0974-4479.2021.00008.3
Bianco A, Venditti A, Foddai S et al (2014) A new problem. Contamination of botanicals by phthalates. Rapid detection tests. Nat Prod Res 28:134–137. https://doi.org/10.1080/14786419.2013.842997
Chaves N, Santiago A, Alías JC (2020) Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 9:76. https://doi.org/10.3390/antiox9010076
Chomchan R, Siripongvutikorn AsstProfDrS P, Rattanapon DP MsR (2016) Investigation of phytochemical constituents, phenolic profiles and antioxidant activities of ricegrass juice compared to wheatgrass juice. Funct Foods Health Dis 6:822. https://doi.org/10.31989/ffhd.v6i12.290
Choudhury FK, Pandey P, Meitei R et al (2022) GC-MS/MS profiling of plant metabolites. Methods Mol Biol Clifton NJ 2396:101–115. https://doi.org/10.1007/978-1-0716-1822-6_9
Davoodbasha M, Edachery B, Nooruddin T et al (2018) An evidence of C16 fatty acid methyl esters extracted from microalga for effective antimicrobial and antioxidant property. Microb Pathog 115:233–238. https://doi.org/10.1016/j.micpath.2017.12.049
Durrett TP, Welti R (2021) The tail of chlorophyll: Fates for phytol. J Biol Chem 296. https://doi.org/10.1016/j.jbc.2021.100802
Elghaffar RYA, Amin BH, Hashem AH, Sehim AE (2022) Promising endophytic Alternaria alternata from leaves of Ziziphus spina-christi: phytochemical analyses, antimicrobial and antioxidant activities. Appl Biochem Biotechnol 194:3984–4001. https://doi.org/10.1007/s12010-022-03959-9
Enikeev AG, Semenov AA, Permyakov AV et al (2019) Biosynthesis of ortho-phtalic acid esters in plant and cell cultures. Appl Biochem Microbiol 55:294–297. https://doi.org/10.1134/S0003683819020066
Fadhel Ali F (2022) Spectroscopic analysis by GC-Mass technique of Citrus maxima leaves and effectiveness of its aqueous extract on some pathogenic bacterial isolates. HIV Nurs 22:1631–1634. https://doi.org/10.31838/hiv22.02.312
Hajam YA, Lone R, Kumar R (2023) Role of plant phenolics against reactive oxygen species (ros) induced oxidative stress and biochemical alterations. In: Lone R, Khan S, Mohammed Al-Sadi A (eds) Plant phenolics in abiotic stress management. Springer Nature, Singapore, pp 125–147
Hao J, Zhu H, Zhang Z et al (2015) Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in-vitro and in-vivo antioxidant activities. J Cereal Sci 64:92–99. https://doi.org/10.1016/j.jcs.2015.05.003
Haque SD, Saha SK, Salma U et al (2019) Antibacterial effect of Aloe vera (Aloe barbadensis) leaf gel against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae. Mymensingh Med J 28:490–496
Huang L, Zhu X, Zhou S et al (2021) Phthalic acid esters: natural sources and biological activities. Toxins 13:495. https://doi.org/10.3390/toxins13070495
Hui BYP, Bhave M, Hwang SS (2019) Potential protective effects of rice seedling extracts of a Malaysian rice variety, Biris, against doxorubicin-induced cytotoxicity. Trop Life Sci Res 30:71–90. https://doi.org/10.21315/tlsr2019.30.2.6
Islam MT, de Alencar MVOB, da Conceição Machado K et al (2015) Phytol in a pharma-medico-stance. Chem Biol Interact 240:60–73. https://doi.org/10.1016/j.cbi.2015.07.010
Jayaraman S, Roy A, Vengadassalapathy S et al (2021) An overview on the therapeutic function of foods enriched with plant sterols in diabetes management. Antioxid Basel Switz 10:1903. https://doi.org/10.3390/antiox10121903
Joshi S, Singh S, Sharma R et al (2023) Gas chromatography-mass spectrometry (GC–MS) profiling of aqueous methanol fraction of Plagiochasma appendiculatum Lehm. & Lindenb. and Sphagnum fimbriatum Wilson for probable antiviral potential. Vegetos 36:87–92. https://doi.org/10.1007/s42535-022-00458-4
Khan Y, Shah S (2022) The phytochemical, pharmacological and medicinal evaluation of quinoa (Chenopodium quinoa Willd). Weed Res 28:141–162. https://doi.org/10.28941/pjwsr.v28i2.1049
Kowalska-Krochmal B, Dudek-Wicher R (2021) The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens 10:165. https://doi.org/10.3390/pathogens10020165
Lima TLC, Souza LBFC, Tavares-Pessoa LCS et al (2020) Phytol-loaded solid lipid nanoparticles as a novel anticandidal nanobiotechnological approach. Pharmaceutics 12:871. https://doi.org/10.3390/pharmaceutics12090871
Mahmoudi S, Nasiri R, Jafari Sales A (2019) In-vitro antibacterial effects of methanolic extract of peppermint (Mentha Piperita Lamiaceae) on standard Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa strain. Jorjani Biomed J 7:4–10. https://doi.org/10.29252/jorjanibiomedj.7.4.4
Martins T, Barros AN, Rosa E, Antunes L (2023) Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: a comprehensive review. Molecules 28:5344. https://doi.org/10.3390/molecules28145344
Mishra V, Tomar S, Yadav P et al (2022) Elemental analysis, phytochemical screening and evaluation of antioxidant, antibacterial and anticancer activity of Pleurotus ostreatus through in-vitro and in-silico approaches. Metabolites 12:821. https://doi.org/10.3390/metabo12090821
Moni SS, Sultan MH, Makeen HA et al (2021) Bioactive principles in exudate gel from the leaf of Aloe fleurentiniorum, traditionally used as folkloric medicine by local people of Aridah and Fayfa mountains, Saudi Arabia. Arab J Chem 14:103400. https://doi.org/10.1016/j.arabjc.2021.103400
Mostafa AA, Al-Askar AA, Almaary KS et al (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 25:361–366. https://doi.org/10.1016/j.sjbs.2017.02.004
Muslim IH, Neamah NF, Shari FH (2024) The Study On Antioxidant And Anti-Inflammatory Effects Of Sodium Copper Chlorophyllin In Male Rats Receiving Indomethacin. Acad Open 9. https://doi.org/10.21070/acopen.9.2024.9223
Nguyen T-T-U, Nguyen P-T, Nguyen T-T et al (2024) Rice leaves or ricegrass—available biomaterial with potential biological activities for different industrial applications: a review. Discov Food 4:106. https://doi.org/10.1007/s44187-024-00187-4
Okpala EO, Onocha PA, Ali MS (2022) Antioxidant activity of phytol dominated stem bark and leaf essential oils of Celtis zenkeri Engl. Trends Phytochem Res 6:137–144. https://doi.org/10.30495/tpr.2022.1952985.1246
Pangi VN, Marukurti A, Reddy AM et al (2023) Anti-vibriocidal activity and gas chromatography mass spectrometry (GC-MS)-based chemical composition of Mirabilis jalapa leaf methanolic extract. J Pharmacogn Phytochem 12:653–662
Patar AK, Bhan S, Syiem D, Sharma A (2016) Ameliorative Effect of Chlorophyllin on Oxidative Stress in Experimental Model of Diabetes. Int J Phytomedicine 8:506–513
Pérez-Gálvez A, Viera I, Roca M (2020) Carotenoids and chlorophylls as antioxidants. Antioxidants 9:505. https://doi.org/10.3390/antiox9060505
Phuoc LH, Suliansyah I, Arlius F et al (2022) Responses of growth and grain yield of IR50404 rice to temperature stress. Int J Agric Sci 6:9–18. https://doi.org/10.25077/ijasc.6.1.9-18.2022
Prasher IB, Manju (2019) Screening of Peniophora nuda (a white rot fungus) for the presence of commercially important bioactive metabolites. Vegetos 32:307–315. https://doi.org/10.1007/s42535-019-00038-z
Rammohan A, Zyryanov GV, Bhagath YB, Manjula K (2023) Chapter Fourteen - Antioxidants: Structure–activity of plant polyphenolics. In: Litwack G (ed) Vitamins and Hormones. Academic Press, pp 395–411
Romodin LA (2024) Chlorophyllin Inhibits Lipid Peroxidation Triggered by the Fenton Reaction. Biophysics 69:1–5. https://doi.org/10.1134/S0006350924700015
Saha M, Bandyopadhyay PK (2020) In-vivo and in-vitro antimicrobial activity of phytol, a diterpene molecule, isolated and characterized from Adhatoda vasica Nees. (Acanthaceae), to control severe bacterial disease of ornamental fish, Carassius auratus, caused by Bacillus licheniformis PKBMS16. Microb Pathog 141:103977. https://doi.org/10.1016/j.micpath.2020.103977
Semenov AA, Enikeev AG, Snetkova LV et al (2016) Ortho-phthalic acid esters in lipophilic extract from the cell culture of Aconitum baicalense Turcz ex Rapaics 1907. Dokl Biochem Biophys 471:421–422. https://doi.org/10.1134/S1607672916060120
Shaaban MT, Ghaly MF, Fahmi SM (2021) Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J Basic Microbiol 61:557–568. https://doi.org/10.1002/jobm.202100061
Syawal H, Hakim L, Effendi I (2020) Phytochemical analysis of Rhizophora apiculata leaf extract and its inhibitory action against Staphylococcus aureus, Aeromonas hydrophila and Pseudomonas aeruginosa. AACL Bioflux 13:2242–2249
Syeda AM, Riazunnisa K (2020) Data on GC-MS analysis, in-vitro anti-oxidant and anti-microbial activity of the Catharanthus roseus and Moringa oleifera leaf extracts. Data Brief 29:105258. https://doi.org/10.1016/j.dib.2020.105258
Taj T, Sultana R, Shahin H et al (2021) Phytol a phytoconstituent, its chemistry and pharmacological actions. GIS-Z Fü Geoinformatik 8:395–406
Tamprasit K, Weerapreeyakul N, Sutthanut K et al (2019) Harvest age effect on phytochemical content of white and black glutinous rice cultivars. Molecules 24:4432. https://doi.org/10.3390/molecules24244432
Thakur M, Singh K, Khedkar R (2020) Chap. 11 - Phytochemicals: Extraction process, safety assessment, toxicological evaluations and regulatory issues. In: Prakash B, (eds)., Functional and preservative properties of phytochemicals.Elsevier, pp 341–361
Thaniarasu R, Abirami S, Kumar TS, Rao MV (2023) Investigation of phytochemicals and antioxidant activity of Plectranthus bourneae Gamble - an endemic plant species of western ghats of Tamil Nadu, India. Vegetos 36:607–614. https://doi.org/10.1007/s42535-022-00424-0
Thepthanee C, Liu C-C, Yu H-S et al (2022) Antioxidant activity and inhibitory effects of black rice leaf on the proliferation of human carcinoma cells. BioMed Res Int 2022:1–17. https://doi.org/10.1155/2022/7270782
Thepthanee C, Liu C-C, Yu H-S et al (2021) Evaluation of phytochemical contents and in vitro antioxidant, anti-inflammatory, and anticancer activities of black rice leaf (Oryza sativa L.) extract and its fractions. Foods 10:2987. https://doi.org/10.3390/foods10122987
Thiemann T (2021) Isolation of phthalates and terephthalates from plant material– natural products or contaminants? Open Chem J 8. https://doi.org/10.2174/1874842202108010001
Toh SC, Lihan S, Bunya SR, Leong SS (2023) In-vitro antimicrobial efficacy of Cassia alata (Linn.) leaves, stem, and root extracts against cellulitis causative agent Staphylococcus aureus. BMC Complement Med Ther 23:85. https://doi.org/10.1186/s12906-023-03914-z
Touyz RM, Camargo LL (2023) Chap. 61 - Reactive oxygen species and oxidative stress. In: Biaggioni I, Browning K, Fink G, (eds)., Primer on the autonomic nervous system (fourth edition). Academic Press, pp 345–352
Ullah B, Hassan S, Khan MN et al (2024) Phytochemical Screening, Antimicrobial, Antipellicle and Antibiofilm Activities of the Root of Alpine Medicinal Plant (Arnebia euchroma (Royle) I.M.Johnst). Pol J Env Stud 33:425–442. https://doi.org/10.15244/pjoes/171298
Vezza T, Canet F, de Marañón AM et al (2020) Phytosterols: nutritional health players in the management of obesity and its related disorders. Antioxid Basel Switz 9:1266. https://doi.org/10.3390/antiox9121266
Wangcharoen W, Phimphilai S (2016) Chlorophyll and total phenolic contents, antioxidant activities and consumer acceptance test of processed grass drinks. J Food Sci Technol 53:4135–4140. https://doi.org/10.1007/s13197-016-2380-z
Yang S-K, Low L-Y, Yap PS-X et al (2018) Plant-derived antimicrobials: insights into mitigation of antimicrobial resistance. Rec Nat Prod 12:295–396. https://doi.org/10.25135/rnp.41.17.09.058
Zainal AA, Salleh NFM, Ahmad WANW et al (2022) Antioxidant properties and antimicrobial effect of Zingiber officinale extract towards Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. IOP Conf Ser Earth Environ Sci 1102:012049. https://doi.org/10.1088/1755-1315/1102/1/012049
Zhang R, Han Y, McClements DJ et al (2022) Production, characterization, delivery, and cholesterol-lowering mechanism of phytosterols: a review. J Agric Food Chem 70:2483–2494. https://doi.org/10.1021/acs.jafc.1c07390
Author Information
Graduate University of Sciences and Technology, Vietnam Academy of Science and Technology, Ha Noi, Vietnam